| |
I had six classes and each conducted their own
experiment on crystals. Most of the classes
designed and conducted their research investigation
on the effects of heat or flame on crystal. The
remainder of this RIP-inquiry description will focus
on the inquiry conducted by one of these classes.
This was the first time that my students are to be
exposed to the process of scientific inquiry in my
classroom and most likely in any of their previous classes. Thus, in order to cover all of the
components of the inquiry process, I expected the
extended guided inquiry to take more than just a few
days. In addition to covering the components of
scientific inquiry, this guided inquiry was designed to
lead students into the study of the characteristics of
the three main types of rocks (a benchmark for the
eight grade science Hawaii Content and Performance
Standards).
Crystals or broken crystals of different kinds
of minerals, or broken pieces of rocks are what
mostly make up rocks. So scientific investigations
focusing on crystals can easily include in their
background information section earth science content
about rocks and minerals. The three types of rocks
can generally be identified by how they are made,
composition and texture.
igneous
  
The surface of the Earth (crust) is mostly made of
igneous rocks-solids made out of crystal which form
directly from the cooling of magma. The formation of
these rocks involves an exothermic process in which
heat is lost. There is also a change from the liquid to
the solid state during the formation of igneous rock.
Some igneous rocks cool rapidly and others slowly,
depending upon how they were formed. Rocks that
typically have a fine grain and appear glassy cooled
rapidly during their formation, while those with large
grainy surface features cooled slowly.
Observing Crystals (Day 1)
I began the unit by providing my students the
opportunity to observe and compare test tubes containing
two different types of crystals: one containing crystals made
from saturated Hawaiian table salt the other containing
crystals made from Epsom salt. The students worked in
groups to perform this task.

Observations were recorded and shared among the
participants within each research group with the goal of
evaluated each one as "subjective" or "objective."

Observations of crystals by my students
(Click on table to enlarge)
Background Information (Day 3)
In class, students shared the background materials
they found for their homework assignment. Students spent
the remainder of the time on Day 3 looking up more
information about crystals in their textbooks and other
resources found in the school library.

Students researching background information at the library
The information gathered by the students was shared,
explained, and written on chart paper for all to see. This
resulted in a general class background information section
for the investigation.

Writing down the information found in background resources

Writing down the information found in background resources
Designing the Study: the Method (Day 5)
Together as a class, the students designed their
scientific investigation, planning what subjects and
materials they would use and the procedures they
would follow. As the students discussed these
components of their study, their thoughts were written
on chart paper at the front of the classroom.
The Subjects
The subjects in this scientific investigation were
salt, sugar, and alum crystals. These crystals were
grown and harvested by the students using the materials
and procedure described below. The crystals that were
used to test the hypothesis in the actual investigation
were then randomly selected using a random numbers table.
The Materials
The materials used for this scientific investigation
are listed below.

In addition, we used a pan balance and graduated cylinders
for massing and measuring chemicals.
Students volunteered to bring the materials necessary
to conduct the investigation to school the next day.
Preparation of Solutions for Crystal Growth (Day 6)
The supersaturated solutions for growing crystals were
prepared following the procedure developed by the students.
This preparation allowed for crystal growth within 2-4 weeks.

Waiting for the water to boil

Massing the chemicals

Using precise measuring tools

Adding alum to the boiling water

Stirring alum into the boiling water to make a super-
saturated solution
|
|
sedimentary
  
Sedimentary rocks are composed of gathering together
of small pieces of pre-existing rocks, dissolved minerals
from the ocean left behind after evaporation, or calcium
(from animal shells or teeth) or other minerals related to
organic processes that come together. Weathering,
decaying of dead organisms, or dissolving from exposure
to flowing water are all processes that contribute to the
breakdown of the original rocks and organic structures that
compose sedimentary rocks. These rocks can usually be
identified by their being made up of many small pieces of
rocks that appear to be cemented together or by their
layered look.
metamorphic
  
 
When the minerals making up any type of rock become
unstable because of environmental forces such as pressure
and/or temperature changes, the rocks themselves change
and are considered to be "metamorphic." These rocks are
usually formed deep inside the earth. Many, but not all, of
these rocks have a sheet-like structure or appear to be
made of stacked plates.
Formulating & Posing a Research Question (Day 2)
Students brainstormed about questions they had about
crystals. The questions were listed on chart paper in the
front of the classroom for all to see. The first step was to
eliminate questions that were not practical (could not lead
to a testable hypothesis and those that were useful (could
lead to a testable hypothesis). Through discussion the
class evaluated each question as good (useable) or bad
(not useable).
The class decided that they would vote on which
question they liked the most and that became their
research question that they they would try to answer
through a scientific investigation. The most popular question
was, What happens to crystals when they're heated?

Student-formulated research questions and the results of
voting (circled in blue)

Students voting for their favorite research question
At the end of the second day of the inquiry, I assigned the
students the homework task of researching information on
crystals.
Constructing the Hypothesis (Day 4)
Using the research question and background information from the previous day, the students worked in groups to answer
the question by writing a testable hypothesis in an "If...then...
because" format. Each student-generated hypothesis included
the conditions that would be used to test it, the predicted
results, and the rationale underlying the prediction.
 Constructing the groups' hypotheses Constructing the groups' hypotheses
Each group of students then listed only the conditions that
would be used to test their hypothesis on the front board so that
these could be examined by the entire class. This way the class
could see all of the experiments that they could choose from for
the class investigation. Voting again took place so that the
students could determine the class investigation that was most
popular. After the winning experiment was determined, all
student were asked to write in their composition books their own
prediction and rationale for that experiment. Each of my six
classes chose a hypothesis. The hypothesis adopted by my
period 6 class was:

The hypothesis to be tested
The Color Scale
I wanted the students to collect both qualitative and
quantitative data for testing their hypothesis. I guided them in
the construction of a scale for crystal color and consistency
changes that would involve both qualitative and quantitative
data. The scale was constructed by the class using background
information and past experiences with the consequences of
applying heat to various substances. They were able to put
their observations in the form of descriptive statements
regarding the heating of objects into a table (see Table 1
below). We then assigned a number to each object to reflect
the amount of change resulting from exposure to a flame. This
became our rating scale (Table 1).
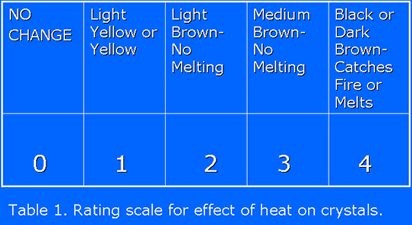
The scale was arranged on a continuum from least (No Change)
to most (Catches Fire or Melts) change. Although one could
argue that these numbers represented ordinal rather than
interval or ratio level data, they permitted my students the
opportunity to perform data summarizing and analysis using
descriptive statistics.
The Procedure

Collecting & Drying the Crystals (Day 7)
The solutions were strained through the coffee filters and
allowed to dry overnight. Crystals were then randomly selected
for the heat study.

Close-up of the alum crystals grown by the students

Close-up of the sugar crystals grown by the students

Close-up of the salt crystals grown by the students
|
|
| |
Our results showed that all three types of crystals
changed color, but some more than others. However, not
all of the crystals melted.

Writing the discussion and drawing conclusions
My students were able to actually see first-hand
through their scientific investigation that different crystals
have different responses to heating and are variably resistant
to changes in form when heated with a flame.
The students' hypothesis was "if we grow salt, sugar,
and alum crystals and heat them, then the crystals will melt
because high heat causes things to melt or burn." This
hypothesis was supported for the alum, but not for the
sugar or salt which did not melt. The sugar did change
|
|
color from white to medium brown, but not consistently. The salt
showed no change in color.
One student wrote:
"In our experiment where we exposed different crystals to
heat, alum was the most affected by heat followed by sugar,
and finally salt with very little change. "
"Standard deviation was calculated for each sample and it
was found that the error bars when graphed did not overlap
which tells us that each average rating is significantly different
from the other graphs."
Another student wrote:
"This hypothesis was partially supported because the salt did
not melt, but alum and sugar melted."
Possible sources of errors that could have influenced
the findings:
"Some possible causes of error could be that each person
heated differently, the lighters produced different temperatures,
or lighters might have been held at different distances. People
might have added different amounts of crystal."
The students were able to use the data obtained in
their investigation to make a decision about their
hypothesis:
"In our experiment where we exposed different crystals to
heat, the alum was most affected by heat, followed by sugar ,
and finally salt with very little change."
|
|
| |
For my first guided inquiry, I had a lot of difficulty
initially coming up with an experiment the students could
do in connection to minerals. Through past experience
and my own experience I felt that the students would find
growing crystals interesting. When I turned it over to them
they did seem interested.
Applying scientific inquiry to my hands-on instruction
was a new experience for me. Because this was my first
attempt, I designed my approach as a mostly guided inquiry,
but tried to encourage student contribution to the process
and creativity when possible.
In the end, my students actually conducted a scientific
investigation that directly compared the behavior of three
types of crystals that were exposed to a flame. Next time,
through socratic questioning, I will guide my students to
discussion and background information that includes the
concept of melting points for different chemicals or minerals.
This will provide them with the knowledge that different
chemicals have different melting points depending upon
what they are made of and how they are constructed.
Hopefully this will lead them into formulating a research
question that will ask a comparison type of question such
as do alum, sugar, and salt behave differently when heated?
Then the student developed hypothesis can include the
prediction and rationale for what is expected to happen
when crystals of the three chemicals are heated and
compared. Guiding students into inquiries focused on
crystal growth rate and/or shape would also directly tie
into the benchmark of how rocks are formed.
I did like how the students were excited about doing
the experiment. It served as a reward to some and an
opportunity to demonstrate responsibility and earn my
trust. When we collected the data all students were
engaged. Whether they were just watching or actually
carrying out the experiment, everyone in every group
knew what was going on and what they were trying to
find out. No students complained about wearing safety
goggles or shoes because they actually saw the importance
of their use. Doing the experiment was also the driving
force behind many of them writing up the beginning part
|
|
of their research report. Thefinal discussion and conclusion
was also easier for them because they understood what the
purpose of the experiment was; they had invested their interest
in the question and hypothesis. Implementation of the RIP ® was undoubtedly useful as a
learning tool and engaging to students. The inquiry approach
could be built upon and understanding strengthened by using
the method to teach each unit. This method of instruction is
very student-centered because it gives students ownership of
their learning and they feel empowered that they have control
in the decision-making that goes into their education. The
students were challenged with the rigor of the RIP approach
as it was their first time carrying out a full investigation using
this process. Repetition and further use will reinforce the
process and improve student understanding.
Traditional lecture and lab activities are comfortable for
teachers and many students. However, this method fails to
engage a person’s curiosity and therefore a deep and invested
interest in their education. While the method of lecture does
offer a direct delivery of content, students aren’t involved in the
research process and it turns into a process of memorization or
for some an opportunity to tune out. The laboratory exercises
are handed to the students where the students are told what
question to ask. When it comes to forming a hypothesis virtually
every student has the same one because they’ve been taught
what to expect. Students are more likely to “fudge” data in
order to get the results they know their instructor is expecting.
Barely anything in the experience resembles the scientific
method. There is no curiosity, there is little room or tolerance
for mistakes which in no way resembles the real world of
science. In a lab, scientists are full of wonder and curiosity.
Most can’t wait to analyze their data to find out if their
hypothesis was correct. Scientists realize that a mistake can
lead to a new discovery…not a failing grade. For this reason the
RIP is invaluable in teaching students the “real” scientific process
where “failure” is okay as long as there is some explanation.
Students are engaging their creative and curious capacities,
which is what causes science to progress. The RIP ® is not just
useful in teaching science. After completing this lesson, and
those that followed, I have found it to be essential. |
|