Chapter thirteen
Sources of plasticity in
behavior and its
physiology: sex, hormones,
environment and the
captivity model
Robert E. Landsman
13.1 COMMENTS ON
BEHAVIORAL PLASTICITY
Behavioral plasticity (or variability) is the
rule, not the exception, and in many instances, environmental perturbations are
a major cause of variability observed in behavior. To the extent that changes
or differences in environmental conditions persist, differences in response
between and/or within members of a single species will persist in both field
and laboratory. Consequently, the scientist who employs behavior as an end
point in his/ her research must carefully assess alternative hypotheses to
explain variability in results, and sometimes the elimination of outliers may
be the elimination of the most valuable findings. Serendipity does not just
occur, it is ferreted out by the reflective investigator.
The
electric organ discharge (EOD) is highly variable, its waveform and frequency
(or rate) being at the mercy of a number of parameters. Certain characteristics
of the EOD are sex and hormone‑dependent and are affected by
maturational, developmental (Chapter 12) and a host of incidental, environmental
factors (e.g. seasons, water quality, captivity). Variability in the EOD even
results from distortion caused by objects close to the fish's body surface,
providing the cues for active electrolocation
(Chapters 5, 17).
304 Sex,
hormones, environment and the captivity model
The variability observed in the EOD emitted by
both the African mormyrids and the South American gymnotiforms is an excellent
indicator of fluctua-tions in these fish's aquatic
habitat and should be considered as a prime example of plasticity and tire
expression of individual variation in a behavior.
This
chapter will focus on the topic of variability in the EOD of weakly electric
fish resulting from factors such as sex, endocrine status, and environmental
perturbations. I will present a rather critical view of the current state of
this field by examining findings, dilemmas, contradictions, and possible resolutions
presented by published data.
Throughout
this chapter, I will use the following abbreviations denoting various hormones:
T (testosterone), DHT (dihydrotestosterone), 11‑KT
(11-ketotestosterone), 17MT (17α‑methyltestosterone, E2
(estradiol), and the steroid hormone precursor CHOL
(cholesterol). Measures of the individual EOD will be referred to as the
duration and/or amplitude of the individual phases of the EOD (P1, P2, P3 and P4), and
the peak power spectrum frequency (PPSF) of the fast Fourier transform
associated with the EOD. As a rule, the shorter the individual EOD, the higher
the PPSF; and conversely, the longer the EOD, the lower the PPSF. However, an
increase or decrease in PPSF may result from changes in the durations of only
specific phases of the EOD: statistically, the PPSF is also more related to the
duration of some phases than to others (Landsman, 1993a,b). Measures of the
rate or pattern of EODs will be referred to as SPIs (Chapter 8).
13.2 SEX‑RELATED
AND HORMONALLY INDUCED EOD
PLASTICITY
Sex differences in EODs have been reported for
both South American gym-notiform and African mormyrid
species. Because the EOD is sensitive to
gonadal hormones, sex‑typical
EODs can be reversed to resemble those of
the opposite sex by gonadal
manipulation and steroid hormone adminis-tration, and
sex differences are highly correlated with season, gonadal maturity
and reproductive state. Generally, the studies that suggest EOD sex
differences were performed in the field, employed small samples, and are
comprised largely of descriptive. non‑statistical accounts of natural sex
dif- ferences in EOD waveform, duration, arid/or SPIs
for several gymnotiform and mormyrid species. These field‑reported sex
differences have rarely been reported in laboratory studies, and if so, only
anecdotally or in one or two species
which were bred in the laboratory. But both laboratory studies (reviews: Meyer, 1983; Bass and Hopkins, 1985;
Meyer et al., 1987;
Mills and Zakon, 1987 Landsman et al., 1990:)
and field studies (Bass and Hopkins, 1983, 1985: Hagedorn and Carr,
1985) have employed hormone
Sex‑related
and hormonally‑induced EOD plasticity 305 305
manipulations to induce male‑ or female‑like
EODs. The majority of the field studies reporting sex differences show
considerable variability and overlap between the sexes. The majority of studies
involving hormone manipulation lack important control groups (i.e. CHOL.‑treated
fish to control for the effects of non‑gonadal steroid hormones, fish
administered blank implants to control for the effects of steroid hormones and
CHOL implants and nonhandled fish to control for the
effects of' all handling including surgical manipulations), and/or many include
control groups of small sample size (e.g. n = 3) composed of both sexes and/or
mixtures of juveniles and adults of both sexes. In many cases, studies used
only methylated androgen, which does not naturally
occur in fish, or DHT, which does not appear to be a major androgen in fish
(although in the guppy, Poecilia
reticulata,
the ability of follicles to synthesize 5 α‑DHT in vitro from
precursors has been demonstrated by Venkatesh et al.,
1991).
Endocrine
studies on weakly electric fish commonly employ the methyl- lated androgen, 17‑MT
(Bass and Hopkins, 1983, 1984; Bass, 1986a;
Landsman and Moller, 1988) which
is more potent than T. Although MT may be used to induce male‑typical
behavior in many fish species, the effects of this hormone are exaggerated and
may be pharmacological in nature. especially when compared with the
effects of T. Landsman and Moller (1988)
implanted MT into juvenile and adult Gnathonemus
petersii and found up to a fivefold
increase in total EOD duration accompanied by large decreases in PPSF from 4100
to 400 Hz! In contrast, Landsman et al. (1990) employed T implants resulting in
plasma levels of T in the range found in
breeding males, and resulting in total EOD duration increases of up
to 33%) with smaller decreases in PPSF to above I kHz (see also Fig. 13.11 ). Problems of hormone dose are
compounded when the effects of MT are
compared with the effects of non‑methylated DHT
or E2 (e.g. Bass and Hopkins, 1983, 1984; Bass, 1986a). Thus, one
must be careful in the interpretation of results obtained with MT
unless substantiated with T or 11- KT, the two predominant androgens found in
fish. DHT was found to be less potent than T in producing behavioral effects on
the EOD, and did not have any effects on PI and P4 in juvenile Gnathonemus petersii (Landsman et al.,
1990). However, the differences in DHT and T may be a dose effect since DHT has
been shown to clear more rapidly than an equivalent dose of T
in other species (discussion: Harding, 1986). Further, many studies on electric fish employed different procedures
for the administration of hormones. including injections, pellet implants
and time‑released silastic implants. Many of the above factors have added
to the variability in findings on sex differences in and hormonal
control of EOD behavior, and have made it particularly difficult to make
generalizations regarding the sensitivity of the EOD to steroid hormones as
well as to make comparisons of steroid effects across and within studies
and species.
306 Sex, hormones, environment and
the captivity model
The
remainder of section 13.2 will focus on those species in which there appears to
be sufficient complementary data to warrant the claim for the existence of sex‑related
EOD differences and their sensitivity to steroid hormones. (When data based on
small samples are cited, the number of subjects is provided.) However, except
for one mormyrid species, Gnathonemus
petersii, the differences between the sexes are overlapping and in many
cases ambiguous: thus, the term 'sexual dimorphism' will not be used to
describe these sex‑related EOD characteristics.
Gymnotiformes
Some gymnotiforms exhibit a sex difference in
their EOD waveform and/or frequency (Chapters 8, 12, 18). Mature female Sternopygus macrurus discharge at higher
frequencies than mature males, while juveniles discharge at frequencies
intermediate between the two (Hopkins, 1972, 1974a; Zakon et al., 1991b; Mills et al., 1992).
When intact S. macrurus were
implanted with silastic capsules containing DHT, the EOD rate decreased and EOD
duration increased significantly compared with both pre‑implant values
and EODs of controls implanted with blanks (Mills and Zakon, 1987: Mills et al., 1992). Two of the authors'
controls, however, also appeared to show consistent changes in EOD frequency
and duration over the experimental period, but in directions opposite to each
other (shown in Mills and Zakon, 1987, figs. 8(b) and 10(b), respectively).
Whether these data reflect effects of the implants themselves is difficult to
interpret as this study did not include a non‑implanted control group. Removal
of the DHT implants from three subjects for 63
days resulted in EOD rates and durations comparable to pre‑implant
values, while three fish with sham removal of' DHT implants retained their
lowest EOD rates and longest durations, suggesting that the steroid effects on
the EOD are not permanent in this species.
A field
study, in which EOD data were recorded and blood samples obtained from the same fish, indicated sex‑specific
relationships between EOD frequency and endogenous steroid levels in S. macrurus
which sug- gested that
androgens, but not E2, modulate EOD frequency in this species (Zakon
et al., 1991b). Males exhibited lower
EOD rates than females, and plasma levels of T and 11‑KT, but not E2,
were inversely related to EOD rate in males, while plasma levels of T and E2
in females were not related to FOD rate (Zakon et al., 1991 b). Interestingly, the EOD sex difference in this
species wits maintained across seasons over which T levels varied in both males
and females, even though males with low levels of androgens had a wide range of
EOD frequencies (Zakon et al., 1991b).
This suggests that (1) the male EOD may be influenced by factors other than
androgen when T and 11‑KT
levels are low (Zakon et al., 1991 b), and (2) the sex difference in
Sex‑related
and hormonally‑induced EOD plasticity 307
EOD characteristics in
this species is maintained by unknown factors in addition to androgen.
Female Sternopygus
dariensis also emit higher EOD rates than males (Meyer,
1983; Chapter 12). Meyer (1983) injected males and females with T, DHT, or E2 in various doses
ranging from 2.5 to 20.0 µg/g body weight.
Following androgen injections, both sexes lowered their EOD rates. Fish with higher pre‑treatment frequencies
showed larger responses to the hormone
treatment than fish discharging at lower frequencies. Unlike S. macrurus, fish treated with E2 showed
frequency effects in the opposite direction
to those treated with androgen.
Further, castrated males showed an 11%
increase and ovariectormized females an 18%) decrease
in EOD rate. Hormone replacement reversed the effects of the surgery, while adminis-tering heterologous
hormones to either sex increased the effects of gonadectomy.
Thus, hormonal effects on the EOD rate of S.
dariensis are not permanent, but rather are activational in
nature. This means that the electric
organ in this species may be bipotential in its
ability to emit male- or female‑like
EODs, and it is likely that the presence or absence of gonadal hormones in adulthood
determines the sexual characteristics of the EOD.
Eigenniannia
virescens (E. lineata: Chapter 18) exhibits a sex difference in EOD rate in the
same direction as Sternopygus (mature
females possess a higher EOD rate than males) but with much overlap between the
sexes (
tures fell within a wide
range of intermediate frequencies. As the fish became ripe, females shifted their
frequency in the upward direction and in many cases surpassed the males' rates
(Westby and Kirschbaum, 1981), suggesting
hormonal involvement in the EOD rate of this species. These incongruent findings suggest at least
three possible explanations: (1) some
of Hopkins' (1974b) fish were gonadally ripe
and producing hormones that influenced EOD rate, (2) the EOD rate is sensitive
to seasonal changes in reproductive
state (page 323), and/or (3) the EIOD rate is affected by cap-tivity (page 336). Sex differences have also been reported
in the waveform and harmonic content of Eigenmannia
EOD activity, with males having a lower ratio of head‑positive to
head‑negative EOD phase durations and a higher content of higher
harmonics (Fig. 13.1; Westby and Kirschbaum,
1981; Kramer, 1985; Kramer and Otto, 1988). The sensitivity of the EOD to steroid hormones has not been adequately studied in this
species to make
any conclusions
regarding the hormone dependence of the sex difference(s), although androgen
injections purportedly decreased the EOD rate (unpubl.
data, cited in Meyer et al., 1987).
Compared with females, mature male Brachyhypopomus occidentalis (formerly Hypopomus) ( a pulse‑type gymnotiform ) have broader tails
308 Sex, hormones, environment and the captivity model
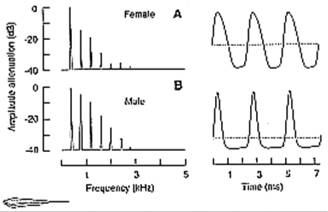
Fig. 13.1 Fourier amplitude spectra (left) and
EODs (right) of female (A) and male (B) Eigenmannia lineata. Notice the lower
ratio of positive to negative EOD phase durations (the identical EOD
frequencies in both sexes seem to be coincidental), and the higher content of
higher harmonics in the male's EOD compared with the female's signal. Zero
potential level is indicated by dotted horizontal line. Modified after Kramer
and Otto (1988).
containing electric organs with larger
electrocytes that produce EODs with smaller PI/112 duration ratios and lower
PPSFs (Hagedorn and Carr, 1985). If the size of the electrocytes accounts for
both tail size and EOD sex differences, then it is not surprising that male
PPSFs were significantly negatively related and female PPSFs positively related
to tail width (Hagedorn and Carr, 1985) (see 'Notes on membrane effects', page
323). When females were injected with 5 pg/g of either DHT or E2,
DHT‑treated fish developed larger, male‑like electrocytes along
with male‑like EODs that were characterized by a significant increase in
the duration of P2 and a 71% decrease in PPSF, while E2‑treated
fish showed no change in electrocytes or EOD (Hagedorn and Carr, 1985).
Mature
female Apteronotus (a gymnotiform with a neurogenic electric organ; Chapters 8,
18) spawned and raised in the laboratory exhibit lower EOD rates than males,
i.e. a sex difference opposite in direction to that shown
in other gymnotiform species, although considerable overlap between the sexes has been reported
(Kirschbaum, 1983; Hagedorn and Heiligenberg, 1985; Meyer et al., 1987). Silastic implants containing estrogen (E2), but not those
containing androgen (DHT), decreased the EOD rate as compared with blank
implant controls (Meyer et al.,
1987). Since non‑handled and CHOL‑implanted controls were not
included, and since all implants
contained less than
0.5 mg of steroid
and the actual dose
Sex‑related
and hormonally‑induced EOD plasticity 309
administered to each
subject is unclear, a more detailed study is needed to make conclusive
statements about the hormonal dependence of the EOD in this species.
Interestingly, Meyer (1984) injected E2, T, α‑ or β‑DHT,
or saline and reported temporary androgen‑induced
in vivo EOD frequency decreases, and in vitro decreases in pacemaker
activity, and no E2 effects. These findings appear to demonstrate
that short‑term EOD hormone sensitivity in this species is due to direct
action of the hormones on the pacemaker. However, saline injection caused both
significant short‑term decreases and increases, and longer‑term
decreases in EOD frequency, although these decreases were significantly smaller
than those caused by androgens. Because injections of saline also influenced
frequency, an accurate interpretation of the steroid data would necessitate a
non‑handled control group, carried through the course of the study,
and/or baseline data collected on the same subjects prior to beginning of the
injection regime for statistical comparison. Also, because EOD data were only
collected over the 7 day injection period, it is difficult to draw conclusions
about long‑term post‑injection hormone effects.
Mormyridae
In their natural habitats, but also on a few
occasions in laboratory‑bred specimens, several mormyrid species appear
to exhibit sex differences that are reflected in temporal (duration) and/or
spectral features (PPSF) of the individual EOD, and sometimes expressed in the
sequence of pulse intervals (SPIs) (Chapters 8 and 12; review: Zakon, 1993).
EODs are steroid sensitive, and so these sex differences can be altered through
administration of steroid hormones in all species investigated (reviews: Bass,
1986a; Landsman et al., 1990;
Landsman, 1993b: Zakon, 1993: Landsman and Moller, in prep). In mormyrids, EOD‑related
sex differences found in the field are elusive under laboratory conditions.
Field studies have indicated that Brienomyrus
brachyistius (long biphasic) (Bass and Hopkins, 1983), Brienomyrus brachyistius (triphasic)
(Bass and Hopkins, 1983, 1985), and possibly Stomatorhinus corneti (Hopkins, 1980; Bass and Hopkins, 1985), S. walkeri (Moller, 1980: Fig. 8.9 (13)) and Hippopotamyrus batesii (triphasic) (one
male and two females: Bass and Hopkins, 1985) may all exhibit sex differences
in EOD waveform and/or duration. Males typically emit EODs that are two to
three times longer (and thus exhibit lower PPSFs) than those of females
(Moller, 1980; Hopkins, 1980, 1981a; Hopkins and Bass, 1981; Bass and Hopkins,
1983, 1985).
The
species identification of Brienomyrus is not
clear. Following the con-vention established in
Chapter 8 (Fig. 8.11), fish studied on location or imported from
310 Sex, hormones, environment and the captivity model
sites in
Although
EOD sex differences have yet to be fully substantiated, the following species
all have steroid‑sensitive EODs: Brienomyrus
sp. and Brienomyrus sp. 2 (Bass
and Hopkins, 1984, 1985; Bass, 1986b; Bass and Volman, 1987), Campylomormyrus tamandua and Hyperopisus bebe (n = 1 fish of each
species; Bass, 1986b), and Stomatorhinus
corneti and Hippopotamyrus batesii (one
fish treated with MT and one with T propionate, respectively) (Bass and
Hopkins, 1985).
The
following subsections contain a more detailed discussion regarding EOD‑related
sex differences and steroid effects in these species as well as in Gnathonemus petersii with an EOD‑related
sexual dimorphism demonstrated in the laboratory.
Brienomyrus brachyistius
(long biphasic) (
Male B.
brachyistius (long biphasic) exhibit
EODs of longer duration with lower PPSFs and different waveforms than females
(Bass and Hopkins, 1983). 17‑MT added to the water induced male‑like
EODs in intact (adult females, and in one juvenile male and female) or
gonadectomized fish (one juvenile male and female, and one adult female) by
increasing the EOD duration twofold with decreases in PPSFs (Fig. 13.2).
Androgen‑induced
effects on the EOD in this species appear to be temporary as the EODs of the
intact androgen‑treated fish reverted to the female type EOD over 24 days
after treatment was terminated by placing the fish in fresh water (Bass and
Hopkins, 1983). Intact (two juvenile males and one adult female) and one
gonadectomized fish implanted with DHT pellets also exhibited male‑like
EODs, while E2 had slight effects on the EOD of immature fish (males
and one female) (Bass and Hopkins, 1983). Surprisingly, E2 treatment
also resulted in a downshift in PPSF and an increase in EOD duration; however,
these changes were not as dramatic as those resulting from androgen treatment
(Bass and Hopkins, 1983). Thus, it is possible that E2 exerts only a
partial masculinizing effect on the EOD because estrogen receptors might not
yet be developed or functional in juvenile fish.
These
findings implicated androgen as a mediator of maleness in the mormyrid EOD, and
suggest that E2 does not have a complete activational, masculinizing
influence on the female EOD. Because DHT cannot be con-verted
to E2 by way of aromatase activity, and because E2 has
some mas-culinizing effects, the extent to which androgen is solely responsible for the
Sex‑related
and hormonally‑induced EOD plasticity 311
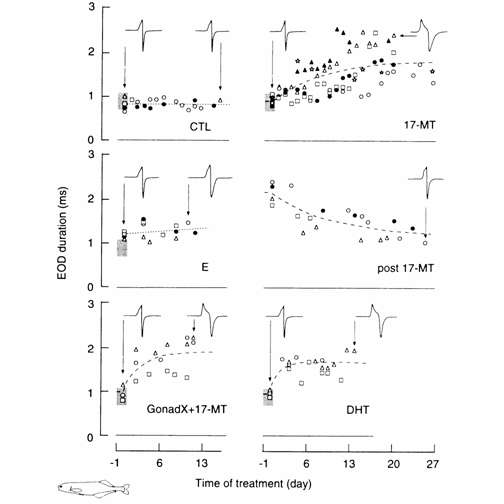
Fig. 13.2 Time course of changes in EOD duration
during hormone treatment periods in Brienornyrus
brachyistius (long biphasic); representative EODs are shown, each symbol is for
one individual. The stippled area to the left of all but one plot is the range
of EOD duration for immature and female fish for one standard deviation (0.161
ms) to either side of the population mean (0.908 ms, n = 25). Dashed lines
represent a least‑squares fit to the straight line (CTL, E) or an
exponential curve (17MT, post 17‑MT, GonadX +
17-MT, DHT). Changes in EOD duration were non‑significant for the non‑treated
control (CTL) and estradiol (E) pellet‑implanted
subjects; but significant when powdered testosterone was added to intact (17‑MT)
or gonadectomized (GonadX + I 7‑MT) fish, and
after it was removed (post I 7‑MT). Thin arrows point to individuals from
which EODs were recorded. Modified after Bass and Hopkins (1983).
expression of
maleness in the EOD of this species is not yet clear. The effect
of CHOL
on the EOD of B. brachyistius (long
biphasic) has not been investi-
gated (compare Bass and Hopkins, 1983, with Bass
and Hopkins, 1985: p. 601).
312 Sex, hormones, environment and the captivity model
Androgen‑specific
receptors have been found in the electric organs of adult mate B. brachyistius
(long biphasic), and a possible sex difference in binding activity was
suggested by preliminary data (Bass et
al., 1984). However, additional assays failed to confirm this result (Bass et al., 1986b). Because a sex difference in androgen binding to receptors in the
cytosol of efectrocytes
could account for sex differences in the EOD waveform, more work in this area
needs to be performed on other mormyrid species. Further, Bass et al. (1986b), using autoradiography,
found 3H‑DHT binding cells in the brain adjacent to the relay
cells of the medullary command nucleus. (These cells
project to the spinal motor neurons that innervate the electrocytes of the
electric organ; Chapter 16.)
Brienomyrus brachyistius
(triphasic) (
The E0D of B.
brachyistius (triphasic)
exhibits sex differences in waveform and duration (Hopkins and Bass, 1981; Bass and Hopkins, 1985). The sexually mature male EOD is
usually double in duration with lower PPSFs and of distinctly different shape
from that of females or juveniles (Fig. 13.3 (A)). However, maleness of the FOD
varies with the size of the fish, and the EOD of large adult females overlaps
with those of males (Bass and Hopkins, 1985).
Bass
and Hopkins (1984) reported that both
androgens, 17‑MT and DHT, induced male‑like EODs in females and
juveniles, expressed in increased duration and decreased PPSFs (Fig. 13.3 (B)).
Surprisingly, E2 pellet implants or injections also increased EOD
duration, lowered peak power, and induced the male‑like waveform shape
(Bass and Hopkins, 1985). B. brachyistius
(triphasic) has not been subjected to treatment
with CHOL or blank implants; it is thus difficult to assess the extent to which
the EOD changes were due to surgery, implants, or general or specific hormone
effects.
Interestingly,
when five fish treated with 17‑MT, dissolved in water, were placed in
fresh water for 25 days, their
hormone‑induced male‑like EODs did not completely revert to pre‑treatment
forms, suggesting that the effects of androgen on the EOD in this species may
be relatively permanent (Bass and Hopkins, 1985). The authors claimed that this permanence is also supported by: ( 1
) the EOD of mature males maintained in captivity for 3 months (n = 3) or 6
months (n = 1) did not revert to the female form (however, only the final day's
EOD is presented; fig. 8 in Bass and
Hopkins, 1985); (2) one transitional male became more male‑like,
and one female with a male‑like EOD (Fig. 13.3) became more female‑like
in captivity: and (3), when one mature male was castrated, its EOD remained
unchanged (male‑like).
Sex‑related
and hormonally‑induced EOD plasticity 313
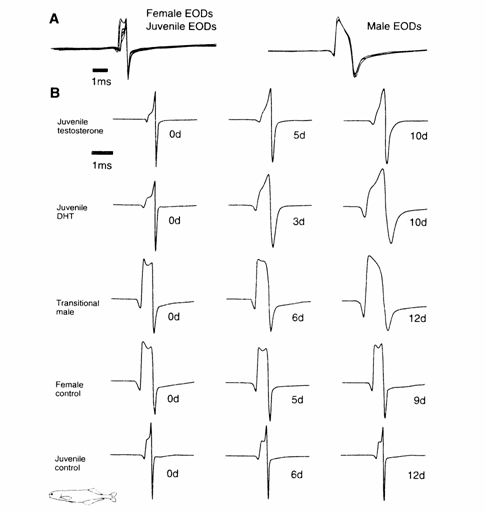
Fig. 13.3 (A) Oscilloscope tracings of EODs from Brienomyrus brachyistius (triphasic) recorded in the field. Notice the differences in
shape and duration between female/ juvenile
(n = 9) and male EODs (n = 3). (B)
EODs of several individuals of B. bra- chyistius (triphasic): juveniles treated with 17α-methyltestostcrone
(juvenile/testos- terone) or 5α‑dihydrotestosterone
(juvenile/DHT) show a change in EOD duration
over 10 days (10d). The EOD duration of a
captive male with a 'transitional' waveform (transitional male)
becomes more male‑like in captivity (6d, 12d). In contrast to all of the above, the
EOD waveform of captive females (female/control) or juveniles (juvenile/control)
remains unchanged when kept in captivity for compare- able times. Interestingly, the
transitional male appears to be more female-like than the female control on 0d, while the EOD
waveforms of neither of the androgen‑treated juveniles assumes the adult
male waveform (A) or the 12d transitional male wave- forms. Note: while the EODs of captive
males and females purportedly become more pronounced (Bass and Hopkins, 1984,
1985: Bass, 1986b), according to the authors, the female control shown did not change in
captivity. Also note that DHT appears to
have a more profound effect than
17‑MT. Modified after Hopkins and Bass (1981) and Bass and
314 Sex, hormones, environment and the captivity model
Brienomyrus sp. (syn. Brienomyrus sp. 2)
(both imported from
EODs from male and female Brienomyrus sp. maintained under laboratory conditions are almost
identical (Bass and Hopkins, 1984). The fish's EOD has
also been subjected to the influence of steroid hormones (Bass and Hopkins,
1984; Bass, 1986b). Bass and Hopkins (1984) reported that 17- MT, DHT, and CHOL, pellet implants lowered
the PPSFs in gonadectomized and intact mates and females, with the effects of
CHOL, being much smaller than those for both androgens. CHOL has also been
shown to have effects on electrocyte
characteristics in the male direction intermediate between controls and T‑treated
fish (Bass et al., 1986b). Thus, a close inspection of methods and results
sections and a comparison of Bass and
Hopkins (1984)
with Bass and Hopkins (1985) does not allow an unambiguous answer about the effects of CHOL, on the EOD
in Brienomyrus sp. For example, in one study (Bass and Hopkins, 1984), CHOL‑implanted
gona-dectomized females (n = 2) showed a 600 Hz drop in
PPSF compared with a 500 Hz drop in gonadectomized controls; while in another
study (Bass et al., 1986a), CHOL‑implanted
intact females (n = 3) exhibited a final average PPSF at least 500 or 600 Hz lower
than non‑treated females and males, respectively. A well‑designed
study with the proper controls should be performed before the conclusion that
"the effects of steroids on the EOD waveform are specific to gonadal
steroids" (Bass and Hopkins, 1985, p. 601) can be made. Although Bass and
Hopkins (1984) concluded that the EODs were also elongated by the steroid
treatments, EODs are presented for only two androgen‑treated fish and no
quantitative data are presented.
Brienomyrus brachyistius
(biphasic) (
B. brachyistius (biphasic) purportedly has no EOD‑related
sex difference (Hopkins, 1980, 1981a). 17‑MT added to the water of three
juvenile males and three adult females did not affect the EOD (Bass and
Hopkins, 1983). This is surprising since no other mormyrid species treated with
androgens has failed to show some hormone‑related
effects on the EOD.
Brienomyrus brachyistius
(imported from
Newly imported B. brachyistius exhibited a statistically significant sex dif-ference in the P2/P3 duration ratio of their EOD, with
males displaying lower ratios than females (Fig. 13.4 (A);
Landsman and Moller, 1991; in prep.). Androgens and CHOL, but not estrogen,
significantly influenced the durations of phases 2 and 3 of the EOD in
laboratory‑maintained fish not exhibiting the EOD‑related sex difference,
with androgen‑treated fish exhi-biting male‑like EODs ( Landsman
and Moller, 1993, in prep. ). Silastic
Sex‑related
and hormonally‑induced EOD plasticity 315
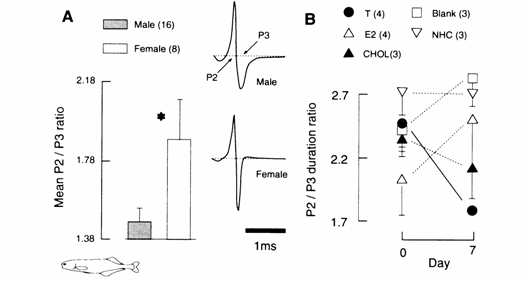
Fig. 13.4 (A) FODs of
male and female Brienomyrus brachyistius
(imported from
implants containing T and 11‑KT (11‑KT:
n = 1 male and 1 female; there was no difference in effects from T and 11‑KT),
17‑MT. and CHOL significantly increased the durations of both phases to
different degrees (Landsman and Moller, unpublished). However, by day 7, only
the two naturally occurring androgens, T and 11‑KT, resulted in a
significant decrease in the P2/P3 duration ratio compared with pre‑implant
ratios and with those of non‑handled or blank‑implanted controls.
Neither E2 nor CHOL exerted any significant effect on the sex‑related
duration ratio, while 17‑MT caused a gradual decrease in this ratio by
day 13 of treatment (Fig. 13.4 (B)).
316 Sex, hormones,
environment and the captivity model
Pollimyrus isidori
The triphasic EOD of P. isidori
exhibits a sex difference in the P1/P3
amplitude ratio (phases 1 and 3 are
positive, phase 2 is negative). Males exhibit a smaller P1 and a larger P3 amplitude, and females a larger P1 and
a smaller P3 amplitude (Fig. 13.5). This results in male ratios being
lower than female ratios (Westby and Kirschbaum, 1982; Bratton and Kramer,
1988; Crawford, 1992). When artificially induced breeding seasons were
introduced, females exhibited amplitude ratios that were threefold larger than
males (Crawford, 1992). Westby and Kirschbaum (1982) and Crawford (1992) found
almost no overlap between the sexes in this ratio, while others (Bratton and
Kramer, 1988) found a largely overlapping, but statistically significant, sex
difference.
While
some reported consistent lower PPSFs for males than females (Westby and
Kirschbaum, 1982), others found extensive overlap of PPSFs and no difference in
EOD duration between the sexes (Bratton and Kramer, 1988; Crawford, 1992).
Alteration of water conductivity conditions eliminated these sex differences
(section 13.3), leading some authors
to conclude that such differences did not function in species or sexual
identification (Bratton and Kramer, 1988). To date, no published studies have
investigated the potential of sensitivity to hormones in this species, so it is
impossible to predict natural sex differences based on hormonal milieu.
Hippopotamyrus batesii
According to Bass and Hopkins (1985), the EOD
waveform of one mature male H. batesii was twice as long
as that for
two mature females, when
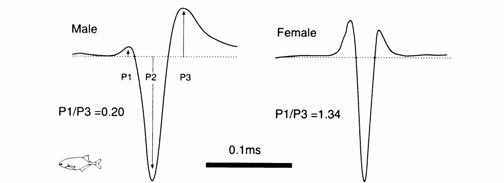
Fig. 13.5 EOD waveform of a male and female Pollimyrus isidori (conductivity: 100 µS/cm). Subjects were selected to demonstrate a presumed sex difference in EOD. P1, first head‑positive phase; P2, head‑negative phase; P3, second head‑positive phase. Note that the ratio of P1 /P3 phase amplitudes is < 1.0 in this male, and > 1.0 in this female. Modified after Bratton and Kramer (1988).
Sex‑related
and hormonally‑induced EOD plasticity 317 317
data were recorded after an unspecified period
of time following capture in
Stomatorhinus corneti
Juvenile S.
corneti undergo a transitional stage
in the development of the adult EOD waveform (Bass and Hopkins, 1985). The
adult male EOD appeared to be longer in duration than that of the female (Fig.
13.6). When juveniles and one female were treated with 17‑MT, or T
propionate (two juveniles, one female), their EODs showed dramatic downshifts
in PPSF and increases in duration, both characteristic of male EODs (Bass and
Hopkins, 1985). However, no cholesterol or blank implants were used, and only
one untreated male served as a control.
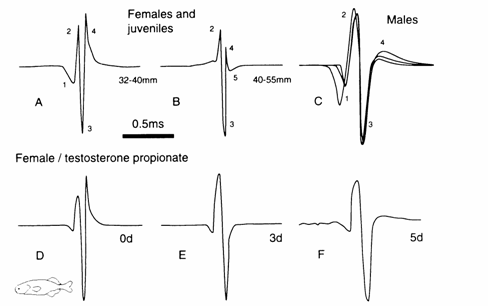
Fig. 13.6 EOD waveforms of Stomatorhinus corneti. Juveniles
and females show two EOD forms depending on total body length (A, B, D). The
EOD of mature males typically has a reduced second positive peak (point 4) and
is longer in duration (C, three EODs superimposed). Testosterone propionate
added to the water of a female induces a mature male‑like EOD over a five
day period (D‑F). Modified after Bass and Hopkins (1985).
318 Sex, hormones, environment and the captivity model
Gnathonemus petersii
Fish obtained during the Nigerian rainy breeding
season and studied on the day they arrived exhibited EOD‑related sexual
dimorphisms: non‑overlapping sex differences in the durations of phases 2
and 3 (the major positive and negative phases) and in the PPSF of the EOD
(Landsman, 1993b). Males exhibited longer durations for both phases and lower
PPSFs than females (Fig. 13.7; Landsman, 1993b). No sex differences were
reflected in the duration of P1, P4, the total EOD or in the duration or
amplitude ratios of P2 to P3.
The
discovery of such a natural sex difference was surprising in light of two
earlier incongruent reports by Kramer and Westby (1985), who did not find a
waveform‑related sex difference (section 13.3), and Landsman et al. (1987), who reported that males
displayed higher PPSFs (and thus shorter EODs!) than females, provided the fish
were unrestrained (section 13.3). These incongruent findings were resolved and
will be discussed in section 13.4. The natural sex differences in the EOD in G. petersii
are consistent with the results of exogenous hormone treatment in this
species (Landsman and Moller, 1988; Landsman et al., 1990).
Androgens
affect the durations of P2 and P3 of the EOD in juvenile and adult G. petersii,
and consequently affect the PPSFs (Fig. 13.8). Landsman and Moller (1988)
and Landsman et al. (1990) demonstrated
that both low and high doses of 17‑MT, T and DHT (administered through
silastic implants) significantly increase the durations of P2 and P3 in both
gonadectomized juveniles (Figs 13.8, 13.9A) and gonadectomized adult males and
females (Fig. 13.9B), while E2 and CHOL have no effect on these
phases (Figs 13.8, 13.9A, low‑ and high‑dose). The androgens 17‑MT
and T decreased the PPSFs in both juveniles (Fig. 13.9A) and adults (Fig.
13.9B), while DHT had no effect on PPSF at low dose and was less potent than T
at the high dose (Fig. 13.9A).
Surprisingly,
E2 caused a slight, but significant, increase in PPSFs in adults
(Fig. 13.9B), but not in juveniles (Fig. 13.9A). This is interesting for a
number of reasons, since it is the only reported E2 effect on the
EOD in mormyrid fish that worked in the direction opposite to that of
androgens. Plasma E2 levels in adults implanted with E2
were about fourfold higher than in juveniles when both were administered the
same dose of this hormone (Landsman et
al., 1990) (Fig. 13.10). This difference in plasma E2 levels in G. petersii
implanted with E2 may have been a function of age-related
differences in hormone metabolism rates (Harding, 1986). Thus, the effects on
the adult EOD not present in juvenile EODs may have been a function of hormone
levels and/or stage of receptor development.
A male‑like
EOD was induced in immature and adult B.
brachyistius (triphasic)
by treatment with E2
( Bass and Hopkins,
1985 )
and, as
Sex‑related and hormonally‑induced
EOD plasticity 319 319
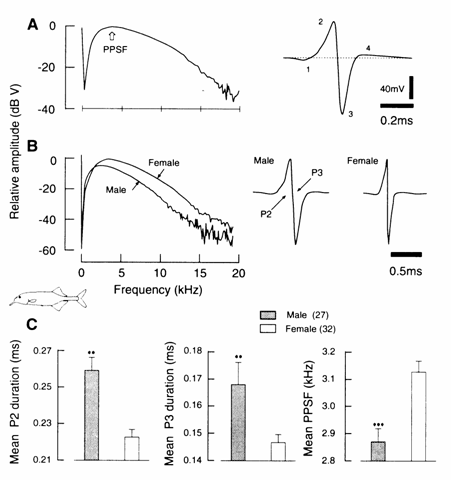
Fig. 13.7 (A) Representative Fourier transform and EOD waveform of Gnathonemus petersii illustrating peak power spectrum frequency
(PPSF) and four EOD phases, respectively. (B) EOD‑related sex difference
in the EOD waveform (right) and associated Fourier spectrum (left) of G. petersii. Data were collected from 27 male and 32 female
adult, gonadally ripe fish on the day they arrived
from
320 Sex, hormones, environment
and the captivity model
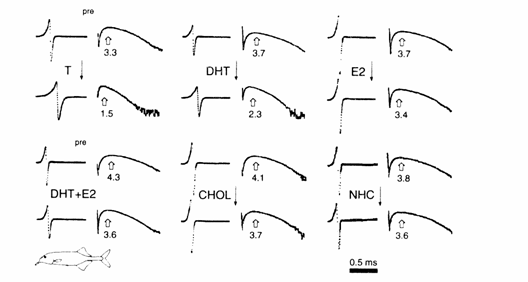
Fig. 13.8 Juvenile EOD waveforms and Fourier
transforms before (pre) and 24 days following gonadectomy
and implantation with high‑dose implants (thin arrows). For explanation
of EOD phases and hormone abbreviations, see Figs 13.7 and 13.9. Note the
increase in duration of phases 2 and 3 and associated decrease in PPSFs (upward
pointing arrows) only in fish treated with T, DHT, or DHT + E2 (for
methods and doses, Landsman et al.,
1990). Modified after Landsman et al.
(1990).
discussed earlier, E2 had a slight,
but not statistically significant, effect on the EOD in the male direction in
immature B. brachyistius (long biphasic) (Bass and Hopkins, 1983).
The
effects of the androgens on P2 occurred
much sooner than those on P3, indicating that the duration of P3 is less
sensitive to androgen, at least in juvenile G.
peterii. This can account for the finding that
males in captivity showed decreases in endogenous androgen levels accompanied
by decreases in the duration of P3, but not P2 (section 13.4). As T levels
decrease, P3 is probably affected before P2, because the latter phase appears
to be extremely responsive to even low doses of androgen.
The
plasma T levels induced by the T implants were comparable to those found in
males just imported during the rainy breeding season (Fig. 13.10; Landsman et al.,
1990). Males imported during the breeding season exhibited longer P2 and P3 durations and lower PPSFs than
females imported at the same time. Newly imported males (caught during their
breeding season) exhibit T levels around 6.0 ng/ml,
while adult males held captive in the laboratory for 5 days exhibit near non‑detectable
levels (Fig. 13.16 (A); Landsman, 1993a,b) similar to those found in controls
(Landsman et al., 1990).
Although
preliminary data suggested that the male‑like characteristics
Sex‑related
and hormonally‑induced EOD plasticity 321 321
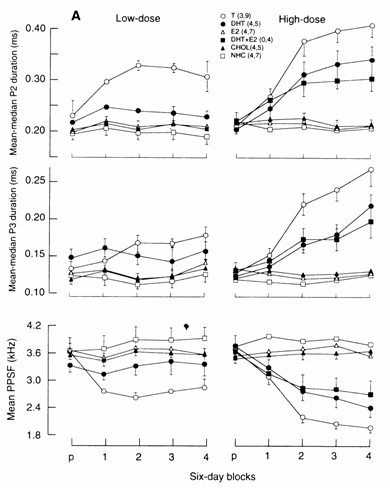
Fig. 13.9 (A)
Effects of steroid hormone silastic implants on the durations of phases 2 (P2)
and 3 (P3) and on the peak power spectrum frequency (PPSF) of the Fourier
transform of the EOD in gonadectomized juvenile and adult Gnathonemus petersii. (A)
juveniles administered low‑dose (left) and high‑dose implants
(right). P2 durations, P3 durations and PPSFs: mean‑median (± SEM) for a
6 day pre‑ (p) and post‑implant blocks (1‑4). (The mean of
the medians was computed because the raw data were skewed.) Treatments: T,
testosterone; DHT, dihydrotestosterone; E2,
estradiol; CHOL, cholesterol; NHC, non‑handled
controls; the two numbers following treatment condition (in parentheses)
indicate sample size in low‑dose and high‑dose studies,
respectively.
in the EOD in G. petersii induced by
male hormones appear to be temporary (Fig. 13.11; Landsman et al., 1990), further studies employing large sample
sizes and proper controls will have to substantiate this possibility.
322 Sex,
hormones, environment and the captivity model
 321
321
Environmentally induced plasticity
323
Notes on
membrane effects
Inter‑ and intraspecific
variability in EOD waveform and duration appear to be a function of variation
in the electrical properties of the electrocyte
membranes (Chapters 8, 16; Bennett, 1971a; Bass, 1986a,b; Mills and Zakon,
1987; Mills et al., 1992; reviews:
Zakon et al., 1991b; Zakon, 1993).
Mills and Zakon (1987) discussed the control and expression of EODs in
gymnotiform and mormyrid fishes based on differences in membrane properties.
The site of hormone action is on the medullary
pacemaker neurons (PMN) and spiking membrane of electrocytes in wave-type fish,
and on the electrocyte membrane (thickening) in pulse‑type
species. The locus of steroid action on the electrocyte
is dependent upon the physiological properties of the electrocytes and thus
varies across species. Steroid‑sensitive sex differences in the
morphology of electrocytes have been reported in a number of species and likely
account for the steroid‑sensitive sex differences found in the EOD (Bass
and Hopkins, 1983; Hagedorn and Carr, 1985; Bass, 1986b; Bass et al., 1986a: Mills and Zakon, 1987:
review: Zakon, 1993). In such species, DHT masculinizes
both the electrocytes and the EOD. Treatment with 17‑MT and 11‑KT
increased the duration of all three phases of the EOD and caused an increase in
the electrocyte width and anterior and posterior face
surface areas in Brienomyrus sp.
(Freedman et al., 1989). Of all
weakly discharging fish studied to date, Sternopygus
macrurus, a wave‑type species,
is the only species in which electrocyte morphology
appears not to be related to the waveform characteristics of the EOD; thus sex
differences in electrocyte morphology cannot account
for the sex differences in EOD waveform and frequency in this species (Mills et al., 1992).
13.3 ENVIRONMENTALLY
INDUCED PLASTICITY
Seasonal
variables
Environmental variables exert a profound
influence on the reproductive biology of
weakly discharging electric fishes. Most influential
are factors
![]()
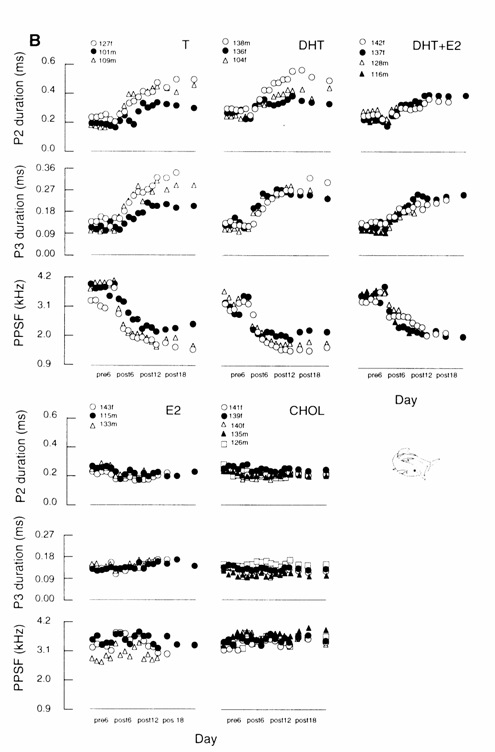
Fig. 13.9 (B) Adult G. petersii. time course
of hormone effects on P2 and P3 durations and on PPSFs for individual males and
females (ID numbers and sex arc indicated) with high‑dose implants across
6 pre‑ and 18 post‑implant days. (Note: this study did not include
low‑dose treated adult subjects.) Only T increased the durations of
either phase at low dose, while high doses of either of the androgens, T or
DHT, increased the durations of both phases 2 and 3 in juveniles and adults.
Only T decreased PPSFs at low dose in juveniles, while high doses of T and DHT
decreased PPSFs in juveniles and adults. E2 caused a slight, but
statistically significant, increase in PPSFs in adults by post‑implant
day 6. Modified after Landsman et al. (1990).
324
Sex, hormones, environment and the
captivity model
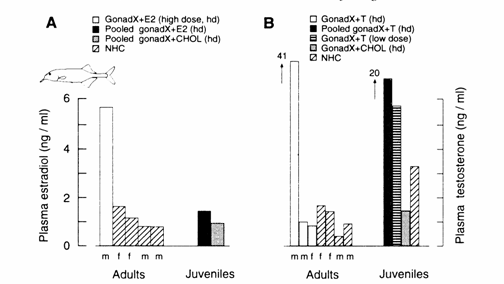
Fig.
13.10 Radioimmunoassay‑determined
blood plasma levels in adult and juvenile Gnathonemus
petersii resulting from low‑dose (1d) and high‑dose (hd) hormone treatments (ordinate: same scale for both
hormones). (A) Pooled and individual plasma estradiol
(E2) levels in gonadectomized (gonadX) E2‑
and cholesterol (CHOL)-implanted fish and in non‑implanted, non‑handled
controls (NHC). (B) Testosterone (T) levels in gonadX
T‑ and CHOL‑implanted subjects and in NHC. Blood was collected from
all fish after 44 days of captivity in the laboratory, 24 days after gonadectomy and implant procedures were performed. (Plasma
E2 levels in pooled NHC juveniles were non‑detectable and are
not shown.) Pooled sample sizes: plasma E2 levels, both E2‑
and CHOL high‑dose implants, n = 5 each; plasma T level, n = 9. Note that
NHC subjects had T and E2 levels comparable to those found in CHOL-implanted
subjects. Also note that NHC adult males compared with the NHC adult females
had lower plasma levels of T and E2. Captivity in the laboratory
probably altered hormone levels (section 13.4). Modified after Landsman (1990).
associated with the
transition from dry to rainy season such as changes in conductivity, water
level and rainfall (Chapter 12).
Gymnotiformes
Sternopygus dariensis collected during the
late dry season in
Environmentally
induced plasticity 325
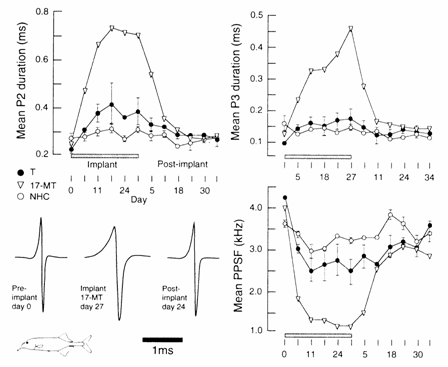
Fig. 13. 11 Androgen
treatment increases the duration of phases 2 (P2) and 3 (P 3) and decreases the
associated peak power spectrum frequency (PPSF) of the EOD in Gnathonemus petersii. Removal of the
hormone‑filled silastic implant results in EOD characteristics resembling
those of pre‑implant data (day 0). Numbers along abscissa indicate day of
recording. Groups: testosterone (T, n = 2 fish), 17 α‑methyltestosterone (17-MT, n = 1) and non‑handled
control (NHC, n = 2). After Voustianiouk and Perrotti
(unpubl.). Note the exaggerated 17‑MT effects
compared with those of T.
S. macrurus breeds during the late dry
season in
Zakon et al. (1991b) determined low to
moderate significant inverse relationships between male plasma T level and EOD
rate in both late dry- season groups (r2 = 0.19 and 0.45,
respectively), and between 11‑K'I' plasma level and EOD rate in only one of the
late dry‑season groups (r2 = 0.23). (r2 values were
calculated from data in Zakon et al.,
1991b.) Males with high T levels
exhibited comparably low EOD rates. Such relationships
326 Sex, hormones, environment and the
captivity model
did
not exist in early dry‑season males, or between T level and rate in
females, and E2 and rate in males or females in all three groups.
The average size of both males and females obtained during the early dry season
was smaller than their same‑sex counterparts obtained during the two late
dry seasons; late dry season gonads were larger and more developed than those
obtained from the early dry season. Androgen levels were directly, and EOD
rates inversely, related to testicular maturity, while ovarian maturity was
directly related to E2 levels, but not EOD rate.
Mormyridae
EOD‑related sexual dimorphisms in G. petersii
were only observed in fish imported during their local breeding (rainy)
season (Fig. 13.12). Male EODs have
longer phases (P2 and P3), and a
lower PPSF than those of females. Estradiol causes a
slight, but statistically significant, increase in PPSF in adult G.
petersii (Landsman et al., 1990). In June, females exhibited their highest PPSFs, which may be
indicative of higher estrogen levels during the breeding season (Fig. 13.12). There was no obvious difference in
testis size between the seasons; ovaries in fish imported
during the pre‑breeding and
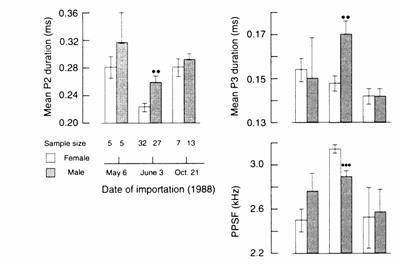
Fig. 13.12
Seasonal sex differences in mean (± SEM) phase 2 (P2) and phase 3 (P3)
durations, and peak power spectrum frequency (PPSF) of the EOD in newly
imported Gnathonemus petersii. Male EOD phases are longer and PPSFs
are lower than females' during the rainy breeding season (June), but not during
the pre‑ (May) or post-rainy (October) seasons. ** Denotes P < 0.001
and *** P < 0.0001 for June male vs. female values. Modified after Landsman
(1991).
Environmentally induced plasticity 327
breeding seasons, however, were more developed
than those examined in fish imported during the post‑rainy season
(Landsman, 1993b).
Similar
to the gymnotiform S. macrurus (Zakon et al., 1991b), sex‑ and seasonally dependent relationships
are evident between various parameters of the EOD and between EOD and physical
characteristics in the mormyrid, G.
petersii (Landsman, 1993b). For example, subjects imported during the rainy
breeding season, but not those imported during the pre‑ and post-rainy
seasons, exhibited relationships between PPSF and the durations of P2 and P3.
And in those subjects, PPSF was statistically more strongly related to P3
duration for females than for males.
Numerous
laboratory investigations designed to examine mormyrid EODs for sex differences
have reported negative findings, non‑reproducible sex differences, or sex
differences which vary from study to study (Hopkins, 1974a; Westby and
Kirschbaum, 1977, 1981, 1982; Lucker and Kramer,
1981; Kramer and Westby, 1985; Landsman et
al., 1987: Bratton and Kramer, 1988). When examining the mormyrid EOD for
sexual differences under laboratory conditions, it is paramount to understand
whether or not, under natural conditions, these differences are permanent or
seasonal. Fish used in laboratory studies are usually obtained from local
sources (importers, pet stores, or local aquaria), and in some instances
collected and imported by the authors. Since, in most cases, no mention is made
as to the season during which the animals were imported or the length of time
they were maintained in the laboratory, it is not surprising that numerous
reports are contradictory and incongruent with field data (reviews: Landsman,
1991, 1993a,b). When gonadal hormones are involved in the control of sexual
behavior of seasonal breeders, ideally, the species' behavior and its
physiological corollaries should be followed through an annual cycle.
Temporal
and spectral characteristics of the EOD are steroid‑sensitive and
therefore indicative of the physiological state of the fish. Thus, the
information conveyed during and outside the breeding season may be quite
different given great differences in gonadal steroid levels. For example during
the breeding season, when hormone levels are high, the duration of specific
phases of the EOD is likely to carry information regarding sexual identity in G. petersii.
When male and female EODs are identical during the non‑breeding
season, species identity, rather than sexual identity, may be their major
function in social signaling.
Water
conductivity
Water
conductivity profoundly affects the fish's electrosensory
motor system (Chapters 5, 8). This section will review the effects of water
conductivity on the purported EOD‑related sex differences.
328 Sex, hormones, environment and the captivity model
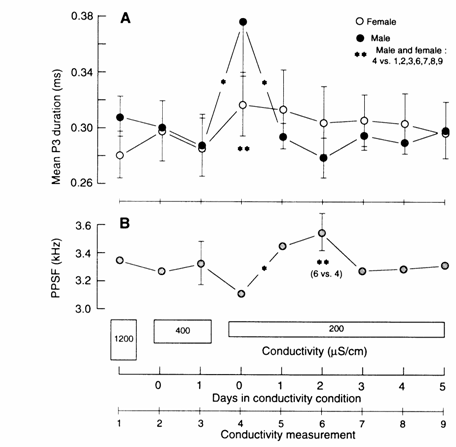
Fig. 13.13 Phase 3 (P3) durations (A) and peak power
spectral frequencies of the Fourier transforms (PPSFs) of associated EODs (B)
for male (n = 5) and female (n = 6) Gnathonemus
petersii across conductivity
measurements (consecutive numbers along x‑axis: 1‑9). (A) Mean (±
SEM) P3 duration: days in conductivity condition are measured immediately prior
to and 1 h following (0) a conductivity change, and then at the 24 h intervals
(1‑5) indicated (x‑axis; days in conductivity condition). Water
conductivity was lowered so as not to disturb the fish by simultaneously
removing portions of the water and adding conditioned low‑conductivity
water to the individual aquaria by siphoning water through plastic tubes
connected to the far ends of the aquaria. The conductivities under which data
were recorded from all fish were (mean + SD): 1200 + 50, 400 +
20 and 200 + 10 µS/cm. A two‑way ANOVA indicated a significant
interaction effect between sex and conductivity (F(8,72) = 2.26, P < 0.05).
Only phase 3 duration of male EODs, not that of females, were significantly
affected immediately following the change from 400 to 200 µS/cm (conductivity
measurement 4) (F(8,72) = 4.91, P < 0.0001). A significant conductivity
condition effect was obtained for P3 duration for all subjects irrespective of
sex (F(8,72) = 3.90, P < 0.001) with the mean duration for conductivity
measurement 4 being longer than the mean durations for all other conductivity
conditions except for measurement 5. No significant conductivity effects were
obtained for phase 2 duration (data not shown).
Environmentally induced plasticity 329
Bratton
and Kramer (1988) exposed male and female P.
isidori to a wide range of
conductivities (3‑500 µS/cm) and reported that the overlapping, but
statistically significant, sex difference in the EOD phase amplitude ratio (P1/P3:
Fig. 13.5) was influenced, but not in all fish. When water con-ductivity was held stable for 24 h at about 100 µS/cm, the male ratio was smaller than that of the
female (section 13.2). Below 100 µS/cm, male and female ratios became more
female‑like (> 1.0), and above 100 µS/cm, male and female ratios
became more male‑like (< 1.0). However, the male and female ratios in
five males and three females that were less than 1.0 were not
influenced by changes in conductivity. The results were the same whether conductivities were varied from low to
high or from high to low. Anecdotal evidence from two males and one female
showed long‑term individual and intraspecific
variability in P1 /P3 ratios. Bratton and Kramer (1988) noted that a decrease
in conductivity caused a decrease in PPSF in
one male, becoming more male‑like
according to the sex difference reported by Westby and Kirschbaum (1982).
Until
the effects of exogenous hormone manipulations and/or endogen- ous levels of
steroid hormones are known for P. isidori, it is difficult to determine
the extent to which variability in the steroid‑sensitive, sex- related characteristics of the EOD is due
to direct conductivity‑induced changes in electrocyte
membrane properties alone or to an interaction
between these effects and
indirect changes in membrane properties induced by physiological factors such as endogenous
hormone levels. Controls such as a non‑manipulated
group and a group subjected to water changes while conductivity was held
constant would have controlled for the effects of conductivity and other
factors on the EOD.
The EOD
of G. petersii exhibits sex differences in the durations of P2 and P3,
and in PPSF. Water conductivity affects EOD‑related sex characteristics
in this species: the duration of P3 (but not P2) and the PPSF (Landsman and
Bowling, unpubl.). The fish were maintained in water
of 1200 = µS/cm for 3 weeks, and subsequently tested in water of (mean +
SD): 1200 + 50, 400 + 20 and 200 + 10 µS/cm.
EODs were recorded immediately prior to and 1 h following
a conductivity change, and again
24 h after
the first
![]()
(B)
Mean (± SEM) PPSFs for all subjects. A two‑way ANOVA indicated a
significant conductivity condition effect (F(8,72) = 2.96, P < 0.01). Mean
PPSFs were significantly higher 1 day (conductivity measurement 5) and 2 days
(measurement 6) following the change from 400 to 200 µS/cm compared with PPSFs
immediately (1 h) following this change (measurement 4). No significant sex or
interaction effects were obtained; statistically significant differences
between means are indicated with * on line connecting the means, while ** below
means indicate significant differences between means for days designated below
stars (PPSF); (α = 0.05). After Landsman and Bowling (unpubl.).
330 Sex, hormones,
environment and the captivity model
change, and every subsequent 24 h for 5 days
following the second change.
Changes
in conductivity interacted with sex, resulting in significant effects on the
duration of P3 and the PPSF (Fig. 13.13). Males, but not females, showed a
significant increase in the duration of P3 immediately following the change in
conductivity from 400 to 200 µS/cm, while by 1 day post‑200 µS/cm, the male
P3 duration decreased significantly, back to its pre‑200 µS/cm level
(Fig. 13.13, top). The same change in conductivity caused an increase in PPSF
for both sexes (Fig. 13.13, bottom).
Seasonal
changes and short‑term environmental perturbations can affect endogenous
hormone levels in mormyrid fish which in turn can cause changes in their
communication signals. Whether the demonstrated conductivity effects argue
against the use of these signals in sexual recognition is currently being
debated (see Bratton and Kramer, 1988, and Crawford, 1992 for two opposing
views). Although the increase in P3 duration and decrease in PPSF in G. petersii, shown under lowered
conductivity conditions, are consistent with the physics of biological
membranes (Bennett, 1971b; Bell et al.,
1976), it does appear that conductivity effects exerted on the EOD are only in
part due to direct physical action on the electrocyte
membrane. The steroid‑sensitive EOD phase 3 in G. petersii (one of the two phases which exhibit sex differences)
is significantly influenced by conductivity. Because environmental
perturbations affect endogenous hormone levels in this species (Landsman, 1991,
1993a), it is possible that variations in conductivity indirectly affect the
EOD by inducing changes in the endogenous hormone milieu. This is supported by
the fact that (1) P3 duration, but not P2 was affected by changes in
conductivity, a finding that was not surprising because the duration of P3
compared with P2 in G. petersii is
more sensitive to changes in androgen levels (Landsman et al., 1990; Landsman, 1991); and (2) environmental perturbations
that influence endogenous androgen levels affected the duration of P3, but not
2 (Landsman, 1991, 1993a). Also, the differential response of P3 in males and
females (Fig. 13.13, top) could be explained by sex‑dependent changes in
endogenous hormone levels resulting from the conductivity manipulations. The
large inter‑ and intra‑individual conductivity‑induced
variability probably reflects what little is known about the interaction
between physical and biological parameters in the determination of the EOD
waveform.
Plasticity
due to capture, handling and confinement
Successful reproduction
requires an organism to synchronize its reproduc‑
tive
physiology and behavior with events in its environment (Moore and Marler, 1988). Consequently, interference with such events or
with the
Environmentally induced plasticity 331
organism's adaptive abilities, possibly through
stress caused by capture, handling and/or confinement, will inhibit
reproduction.
Changes
in environmental factors and laboratory manipulations are known to affect
reproduction and alter the sex‑related characteristics of the EODs in
electric fish. This is probably why it is difficult to breed these fish in
captivity. By manipulating environmental variables, however, several authors
were able to induce a few species to breed in the laboratory (Chapter 12).
Because sex differences in EODs are rarely seen in any wild-caught fish
maintained under 'typical' laboratory conditions, it appears that the variables
removed or introduced by bringing electric fish into captivity inhibit reproduction,
at least in part, by changing their behavior. Consequently, while there are
numerous field studies in which sex differences in the EODs of a number of
species were observed, in only one species to my knowledge has a completely nonoverlapping unambiguous EOD sex difference been
reported in feral animals imported and brought into the laboratory (Landsman,
1991, 1993a).
In
gymnotiforms, female Brachyhypopomus occidentalis (formerly
Hypopo-mus) injected with saline as a control for hormone administration
increased their PPSFs (Hagedorn and Carr, 1985), and Sternopygus dariensis of both sexes
exhibited increases in EOD frequencies following handling compared with a non‑handled
control group (Meyer, 1983).
Landsman
et al. (1987) reported that a sex
difference in the waveform and possibly also duration of the EOD in the
mormyrid, G. petersii, was eliminated when males and females were either
confined or restrained (Fig. 13.14). When the same fish, however, were
permitted to rest freely, males exhibited higher PPSFs than females (Fig. 13.14
(B)).
Restraints
were used to reduce individual variation due to fish movement. In the
'confined' and 'restrained' conditions, both sexes emitted highly variable EODs
which deviated from the normal PPSF range for either sex, suggesting that under
aversive conditions, the EOD variations may reflect a change in message
analogous to the alarm calls exhibited by other vertebrate species (Fig. 13.14
(C)). There was also a dramatic decrease in variability between individual
PPSFs for both males and females in the 'free' condition compared with the
relatively large variability between individuals in the 'restrained' condition
(Fig. 13.14 (A)). Thus, it appears that a sex difference in mean PPSFs was
eliminated by the high variability in the ‘restrained' condition, which
resulted in greater overlap among individual male and female means.
These
results suggest that EOD behavior is extremely malleable when fish are
subjected to procedures that have been shown to be stressful, such as
confinement and restraint. Stress in fish is typically assessed by measuring
blood corticosteroid levels. Fish, similar to mammals, respond to exposure to
stress with an
increase in circulating corticosteroids and catecholamines
332 Sex, hormones, environment and the captivity model
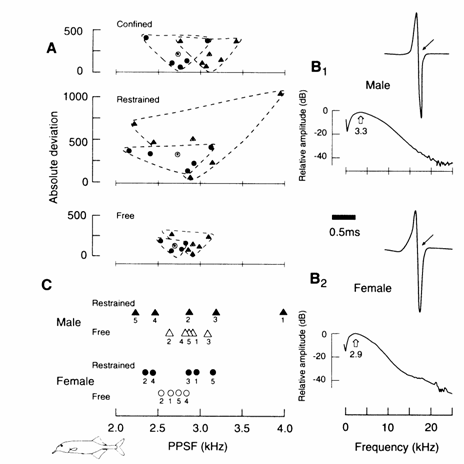
Fig. 13.14 Effects of confinement and restraint on
the EOD in male and female Gnathonemus petersii. (A) EODs recorded
from the same fish under 'confined', 're-
strained' and 'free' conditions (temperature, 22.5oC;
conductivity, 150 µS/cm). Absolute deviations (absolute difference
between each subject's mean PPSF and the
group mean for that sex)
indicating the variability of individual average peak power spectral frequencies (PPSFs) (y‑axis),
are plotted as a function of the individual average PPSFs (x‑axis). Each
group mean (absolute deviation) is plotted as a function of its group mean PPSF. Black circles
represent female individual means; open circles with point inside represent female group
means; black triangles represent male in-
dividual means; open triangles with point
inside represent male group means. The enclosure surrounding each sex group
distribution illustrates the amount of variability of individual mean PPSFs.
Each fish was confined in a suspended gauze envelope allowing some lateral
mobility. After 15 min, for each fish, a mean PPSF was calculated from six
separate readings. 'Confined' males tended to have higher PPSFs than females
(means + SE = 3102.4 + 110.4 Hz and 2739.8 + 117.8 Hz, respectively); however, this was only a trend,
t(8) = 2.25, P < 0.06. The great variability between fish (illustrated by
the vertical spread of the male and female distributions shown in (A), top)
suggested the recording technique (which allowed the fish some degree of
mobility) to be the source of the observed variability, ob- scuring
any sex difference. Therefore, following a 5 day resting period, the fish were again individually placed into the test tank, this time either 'restrained'
within the
Environmentally induced plasticity 333
as well as a change in gonadal hormone milieu
(e.g. handling, confinement and restraint: Strange et al., 1977; Mazeaud et al., 1977; Mazeaud and Mazeaud, 1981; Pickering
et al., 1987; Safford and Thomas, 1987; Carragher
and Pankhurst, 1991; pollutants: Ilan and
Yaron, 1983: saline injection: Hegab and Hanke, 1986).
Gonadal
steroid levels have effects on EOD rate and waveform duration and shape.
Environmental influences on the EOD could be exerted through changes in plasma
levels of gonadal and/or interrenal (stress) hormones. For example, cortisol could
possibly affect the EOD either directly by influencing the electric organ
electrocytes or indirectly by influencing gonadal steroid hormone levels. In
freshwater tilapia (Sarotherodon mossambicus) and rainbow trout (Oncorhynchus mykiss, formerly Salmo gairdneri), increased
cortisol has been shown to augment the entrance of sodium,
calcium and
![]()
gauze
envelope tied to a vertical support within the tank or 'free' to leave or
return to a porous porcelain shelter tube. The 'restrained' condition deprived
the fish of all locomotor activity; in the 'free'
condition, the fish were permitted to move about freely and discharge
recordings were taken only when a fish remained in its shelter. Because the
'restrained' and 'free' conditions were introduced following analysis of data
from the initial 'confined' condition, fish were assigned to the two treatments
in a counterbalanced order so that each condition was separated by a 5 day rest
period. Restraining the fish did not result in the expected sex difference in
average PPSFs, t(8) = 0.54, P = 0.60; there was great overlap in individual
male and female means ((A), middle). Surprisingly, in the 'free' condition
((A), bottom), the male PPSF was located at a significantly higher frequency
(mean + SE = 2902.6 + 73.1 Hz) than the female PPSF (mean +
SE = 2691.5 + 60.6 Hz), t(7) = 2.14, P < 0.05, one-tailed; point biserial correlation coefficient: rpb
= 0.63. Levene's test (Keppel, 1982), to assess
differences in variability (absolute deviations), revealed a significant
treatment effect, F(1, 7) = 6.15, P < 0.05, indicating greater variability
between the individual means in the 'restrained' condition (mean absolute
deviations were 492.8 for males and 324.5 for females) compared with the 'free'
condition (male mean = 104.2, female mean = 93.5). Note the trend toward the
expression of a sex difference and the intermediate variability of individual
means in the 'confined' condition ((A), top) compared with the 'restrained' and
'free' conditions ((A), middle, bottom).
(B)
EODs from representative G. petersii
in the 'free' condition showing a male (B1) and a female (B2))
differing in PPSF (3.3 vs. 2.9 kHz) and EOD duration (shorter P3 duration in
the male: arrows).
(C) Fate of individual mean PPSFs for male and female Gnathonemus petersii under 'free' and 'restrained' conditions. Under the 'free' condition, both sexes exhibited PPSFs between 2540 and 3100 Hz (males: 2660 to 3100 Hz; females: 2540 to 2820 Hz). Under 'restrained' conditions, however, individuals shifted their PPSFs either to the low end of the spectrum (males: 2230 to 2450 Hz; females: 2370 to 2412 Hz) or to the higher end (males: 2875 to 3950 Hz; females: 2867 to 3140 Hz). Under the 'free' condition, PPSFs are clustered; under 'restrained' conditions, PPSFs are dispersed to either end of the spectrum. Numerals identify individual fish. Modified after Landsman et al. (1987 and unpubl.).
334 Sex, hormones, environment and
the captivity model
chloride within muscle cells and to support
retention of inter‑ and intra-cellular water (Assem
and Hanke, 1981). Thus, cortisol has been implicated
in the regulation of cellular ion channels. A similar type of regulation may
occur at the electrocyte level in the electric fish
and could possibly account for environmental effects on the EOD (see also
Ferrari and Zakon, 1993).
Behavioral
changes due to stress have been demonstrated in a number of other fish. Such
changes may be related to the hormonal changes which typically accompany stress
manipulations.
When
pumpkin seed sunfish (Lepomis gibbosus) were confined in small quarters, they
established and defended territories (Erickson, 1967). The author found a
negative relationship between aggressive behavior and interrenal
volume, The highest‑ranking swordtails (X. helleri) showed the least adrenocortical
activity as measured by the nuclear diameter of adrenocortical
cells (Scott and Currie, 1980). (However, Scott and Rennie,
1980, found that the nuclear diameter is only an approximate indicator of
plasma cortisol level in Coregonus lavaretus.) Dominant Oncorhynchus mykiss (Salmo gairdneri) also exhibited lower interrenal activity
than subordinates (Noakes and Leatherland,
1977). Most subordinate
Manipulations
such as the confinement procedures used on G.
petersii may
have constituted stressful events and resulted in a hormonal 'stress response'
that, in turn, altered the EOD (Landsman et
al., 1987). It seems unlikely that the stress
hormone, cortisol, was responsible for these effects,
Environmentally induced plasticity 335
because G.
petersii implanted with open‑ended silastic capsules containing this
hormone did not exhibit changes in EOD characteristics (Landsman, unpubl.). (Cortisol levels have not been measured in weakly
electric fish.)
Steroid
hormones other than corticosteroids have also been implicated in the hormonal
'stress response' in fish. Transfer from a 'home' container to crowded
conditions resulted in large increases in DHT, E2, and estrone 1‑3 h later in the sea lamprey (Petromyzon marinus) and yellow eel (Anguilla rostrata) (Epple et al., 1982). Progesterone (P) and T levels dropped within the
first hour and then rose sharply, while androstenedione
was not detectable. In pre‑spawning adult lampreys of both sexes, androstenedione titers increased in response to surgery,
agitation, decapitation, and anesthesia followed by decapitation. T levels
increased only after surgery, and cortisol increased only after agitation. E2
titers fell after surgery, while estrone did
not change. The authors concluded that their results suggested that in
vertebrates many steroid hormones are ‘stress hormones'. However, the data
could also be interpreted as indicating that only agitation, the manipulation
that increased cortisol, results in a stress response in these species. When
spotted sea trout were maintained in captivity, after a 1 day post-capture
decrease in plasma levels of T and E2, T levels and gonadosomal index (GSI) for males did not change, while GSI
values for females decreased; cortisol levels in females increased by day 1
post‑capture, but returned to initial levels by day 21 (Safford and
Thomas, 1987).
Chronic
confinement for 1month as well as acute handling stress resulted in suppression
of plasma levels of both T and 11‑KT in sexually mature male brown trout
(Pickering et al., 1987). Exposure of
brook trout to cadmium resulted in elevated plasma androgen levels, whereas
crude petroleum caused suppression of androgens in salmon and flounder
(Truscott et al., 1983).
Hannes and Franck (1983) measured blood androgen and glucocorticoid levels in socially isolated and non‑isolated
male Haplochromis burtoni and Xiphophorus helleri. Social‑living
fish of both species exhibited significantly
higher mean
concentrations of both androgens and corticoids, with no
relationship between the
levels of the two hormones. Thus, social isolation
reduced circulating androgens, but not as a result of isolation
stress
because corticoid levels also fell. Social isolation of Sarotherodon mossambi-
cus resulted in reduced
gonad weight, spermatocyte/spermatogonial ratio,
and size of interstitial cell nuclei (Silverman, 1978), all of which are regulated by
hypothalamic and pituitary activity.
As sex differences in EOD behavior can be
altered by sex‑hormone manipulations, it is likely that the effects on
the EOD‑related sex characters caused by environmental variations and
laboratory manipulations result from
changes in endogenous gonadal hormone levels. Whether or not such hormone
changes constitute a stress response is
not clear.
336 Sex, hormones, environment and
the captivity model
13.4 CAPTIVITY‑INDUCED PLASTICITY: THE
MORMYRID
CAPTIVITY MODEL
Captivity
and reproduction
Environmental variation plays a role in electrocommunication. Here I will focus on the discrepancy
between data on EOD‑related sex‑differences collected from fish in
their natural habitats on location, and those collected from wild‑caught
specimens brought into the laboratory, as well as on between ‑laboratory
discrepancies.
Our
understanding of the mechanisms underlying the influence of captivity on
reproduction has been limited because no adequate animal model has been
identified in which to study the effects of captivity on both reproductive
behavior and its underlying physiology. This is largely due to the fact that
most feral animals brought into captivity fail to exhibit any sexual behavior
(Moore and Miller, 1984). Manipulations that cause stress‑responses and
the effects of captivity disrupt reproduction across a wide variety of species.
Laboratory manipulations (such as food deprivation, overcrowding, extensive handling,
isolation, extreme temperatures, surgical procedures and injections) have
demonstrated that severe stressors inhibit reproductive behaviors (e.g. Moore et al, 1982; Zoeller
and Moore, 1982; Miller and Moore, 1983; Moore and Miller, 1983, 1984). To the
extent that such reproductive behaviors are hormone dependent, it is generally
assumed that the endogenous hormone changes resulting from such stress
manipulations cause the behavioral change. However, these laboratory
experiments employ severe manipulations and leave the animal no recourse other
than a severe stress response (Wingfield, 1988).
Feral animals captured and maintained in the laboratory also exhibit changes in
reproductive physiology (e.g. in mammals, Rivier et al., 1986; in birds, Wingfield, 1988; in amphibians, Moore and Deviche, 1988; in fish, Mazeaud
and Mazeaud, 1981) and in reproductive behaviors
(Elton, 1979; Erwin and Deni, 1979). However, little is known regarding the
link between captivity, endogenous hormone milieu, and reproductive behavior in
wild‑caught animals (Moore and Miller, 1984).
It has
always been assumed, but not demonstrated until recently (Landsman, 1991, 1993a),
that some of the same hormonal changes that accompany laboratory stress-responses
are responsible for the captivity effects on behavior and reproduction. Because
various characteristics of the EOD behavior reflect physiological state in
electric fish (e.g. endogenous hormone milieu), this behavior may be an
excellent indicator for captivity effects. Electric fish may therefore serve as
an excellent model in which to study the effects of captivity on reproductive
behavioral physiology.
Captivity‑induced
plasticity: the mormyrid captivity model 337
Captivity
and mormyrid EODs
Anecdotal evidence based on only a few fish
suggests that the EOD is altered over time in captivity. When two control
female S. corneti were held captive
in the field for 6 to 14 days, their average PPSFs decreased in the direction
exhibited by males or androgen‑treated females (Bass and Hopkins, 1985).
But these changes in peak frequency were not statistically significant and were
well within the range of normal female EODs (Bass and Hopkins, 1985). However,
the EODs of mature males maintained in captivity for periods of 3 to 6 months
did not revert to a female‑type waveform; in fact, after 12 days in
captivity, one male with a 'transitional waveform' exhibited a more male‑like
PPSF. These authors suggested that the EODs of adult males are permanent, but
have a modifiable appearance based on an individual's overall physiological
state. Similarly, the EODs of captive male and female B. brachyistius (triphasic) became more
pronounced, i.e. more male‑like and more female‑like, respectively,
while those emitted by juveniles did not change (Bass and Hopkins, 1984, 1985;
Bass, 1986b). In contrast, Bass (1986b) reported the opposite effect in one
captive adult male B. brachyistius
(long biphasic) that exhibited a reversal to the natural female waveform over
time.
Incongruent
findings regarding EOD‑related sex differences have been reported across
studies and appear to be a result of captivity effects. For example, in G. petersii, only freshly imported rainy‑breeding‑season
fish exhibited sex differences in the direction predicted by results of studies
employing exogenous hormone manipulations (Landsman and Moller, 1988; Landsman et al., 1990). Fish imported and then
maintained in the laboratory for periods of 3 to 4 weeks exhibited either a sex
difference in the opposite direction to that predicted by hormone treatment
effects (Landsman et al., 1987) or no
sex difference at all (Kramer and Westby, 1985).
Mormyrid
captivity model
Landsman and Moller (in prep.) propose that a
suitable model for the study of the effects of captivity on behavior and
its physiological substrates would be a species that, both prior to and
following its introduction into the laboratory, (1) exhibits an easily
quantifiable overt behavior that clearly changes as a function of laboratory
confinement, and (2) allows an equally easy access to and measurement of
potential underlying physiological causes.
Weakly electric fish seem to meet the requirements of such a model: they can be obtained from local fish
importers; their EOD behavior is readily accessible with the use of
recording electrodes; the EODs can then be quantified and analyzed with
standard electrophysiological techniques (e.g. using
oscilloscope displays, personal computers, spectrum analyzers).
338 Sex, hormones,
environment and the captivity model
These fish continually discharge under both
field and laboratory conditions.
Mormyrids.
in general, exhibit sex differences in their EODs that are observable in field
but not laboratory studies. Even studies on the same or different species
performed within and across laboratories have reported incongruent results
regarding such EOD sex differences (review: Landsman, 1991). The EOD is
extremely susceptible to environmental perturbations, as indicated by studies
reviewed in the previous section. Research on two mormyrid species, G. petersii
(Landsman, 1991, 1993a) and B. brachyistius (Landsman and Moller, 1993;
unpublished) has experimentally demonstrated the profound effects that
captivity exerts on the EOD, and can thus explain the variability and incongruences reported in previous studies focusing on EOD‑related
sex differences.
Within
2 h of arrival on the day that subjects were received from
The
finding that sex‑related characteristics of the EOD change as a function of the amount of time in captivity
can account for the incongruent results reported in previous work on EOD‑related
sex differences in G. petersii. In fish that had been
maintained in the laboratory for approxi- mately 4 weeks prior to data collection (Kramer, pers. communication,
1986), Kramer and Westby (1985)
did not find any sex difference. In fish
that were maintained in iso‑sexual groups for approximately 3 weeks prior to data collection, Landsman et al. (1987) reported that male G. petersii
exhibited higher PPSFs and shorter EODs than females, i.e. a sex difference
opposite in direction
to those found in field studies in related species
Captivity‑induced
plasticity: the mormyrid captivity model 339
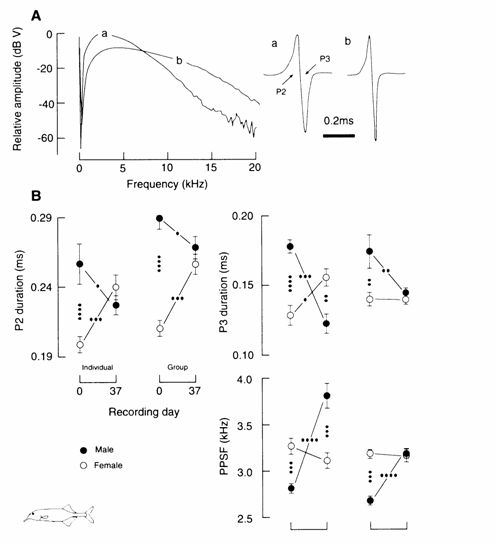
Fig. 13.15 Effects of captivity in the laboratory on
sex differences in EOD phase durations and EOD‑associated PPSFs in Gnathonemus petersii. (A) A Fourier
transform and EOD recorded from a male on 'Day 0' (a) and from the same male
after 37 days of laboratory captivity (b). Note decrease in EOD duration and
shift in power spectrum following the captive period. Arrows and numbers refer
to phases 2 and 3. (B) Quantitative data: means (± SEMs)
of phases 2 (P2) and 3 (P3) durations, and PPSFs (males: n = 18; females: n =
17) for fish maintained in individual tanks (left panels; n = 7 males and 7
females), or group aquaria (right panels) with fish of the same sex (n = 7
males and 7 females) or mixed sexes (n = 4 males and 3 females). (Group data
were pooled.) Asterisks (* P < 0.05; ** P < 0.0 1; *** P < 0.005; ****
P < 0.0005) indicate significant sex differences (vertical) and significant
differences in means for each sex between days 0 and 37 (horizontal),
respectively. All sex differences were either reversed or abolished by the end
of the captivity period regardless of housing conditions. Phase 3 durations and
PPSFs of males appear to be more affected by captivity than those of females
(details of methods and statistical analyses: Landsman, 1991, 1 993a,b).
Modified after Landsman (1991, 1993a).
340 Sex, hormones, environment
and the captivity model
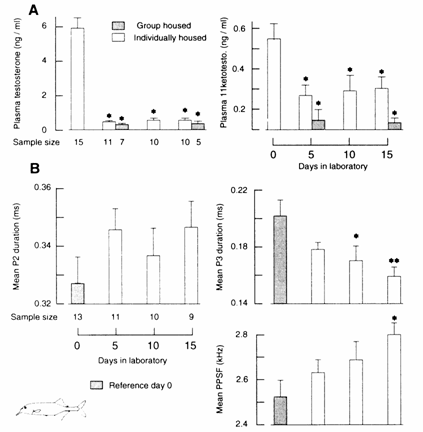
Fig. 13.16 Simultaneous effects of laboratory captivity
on (A) plasma androgen levels, and (B) EODs in sexually mature male Gnathonemus petersii obtained from
(reviews: Bass, 1986a; Hopkins, 1986a; Kramer,
1990a), and opposite to those predicted
by steroid hormone treatment (Landsman and Moller, 1988; Landsman et al., 1990). Finally, a non‑overlapping sex difference was
found in newly imported fish, with males
exhibiting lower PPSFs and
Captivity‑induced plasticity: the mormyrid captivity model 341
longer P2 and P3 durations than females
(Landsman, 1993b), characteristics congruent with those found in hormone
studies on this species and hormone and field studies on related species.
The
effects of captivity on the EOD in G.
petersii appear to be due to factors
other than those considered to be 'stress‑related'. The subjects
maintained in individual aquaria showed more dramatic captivity‑related
EOD effects than those shown by subjects maintained in groups (Fig. 13.15 (B)). Subjects maintained in groups in
this and in previous studies establish dominance hierarchies which involve much
aggression (Bell et al., 1974;
Kramer, 1976a,b; Crockett, 1986). The subjects maintained in group aquaria
displayed much biting and chasing over the entire experimental period, while
individually housed subjects appeared calm and, when not scavenging for food,
were at rest in their shelters (Landsman, 1993a). It would appear then that the
subjects maintained in groups were more ‘stressed' than those maintained in the
individual aquaria.
Further,
as mentioned earlier, cortisol implants had no effects on the durations of
individual EOD phases or the PPSF. In the same study, control fish implanted
with cholesterol exhibited more variability in EOD characteristics than fish
implanted with cortisol. This could, however, indicate a ceiling effect of
cortisol on the EOD as it is possible that cortisol levels were already
elevated in these subjects, and remained at high levels in the cortisol‑implanted
fish.
Testing
the mormyrid captivity model
The EOD‑related sex difference in Brienomyrus brachyistius (originating
from
The
effects of captivity on the EOD in G.
petersii and B. brachyistius can
explain a number of inconsistencies regarding sex differences reported within and across studies involving electric
fish. For example, in one study, female E.
virescens were reported to discharge
at higher frequencies than mature males (
342 Sex, hormones,
environment and the captivity model
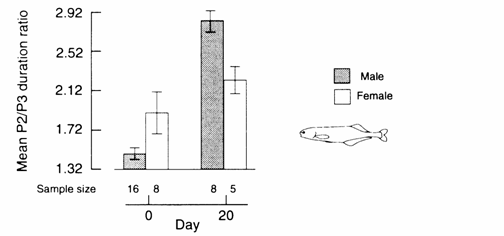
Fig.
13.17 Effects of captivity on the phase 2/phase 3
(P2/P3) duration ratio sex difference in the EOD of adult Brienomyrus brachyistius.
On 'day 0' (day of import from
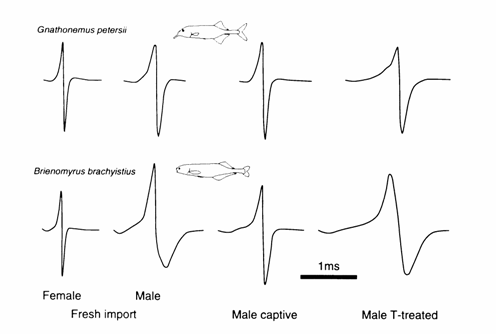
Fig. 13.18 Influences of captivity and testosterone
(T) on the EOD waveform in male Gnathonemus
petersii and Brienomyrus brachyistius
(imported from
Captivity‑induced
plasticity: the mormyrid captivity model 343
EOD rate in communication of sexual identity. It
is not clear how long the fish were maintained in the laboratory prior to the
pre‑rainy season data collection. Further, post-rainy season data were
collected between 18 and 62 days following the pre‑rainy season data
collection. Because the EOD rate in this species is steroid sensitive (Leong, unpubl. data, in Meyer et al., 1987) and a control group (not
exposed to the rainy season condition) was not included, it is equally
parsimonious to explain the EOD changes in terms of captivity effects. In
another study, at the time of spawning, male E. virescens EOD rates
were always lower than female rates (Hagedorn and Heiligenberg, 1985). First‑generation
animals spawned more readily and regularly than their wild‑caught
parents, indicative of captivity‑induced suppression of reproductive
processes when feral animals are brought into the laboratory.
A sex
difference in the negative phase (P2) of the EOD in P. isidori (Westby and
Kirschbaum, 1977) could not be replicated (Westby and Kirschbaum, 1982). The
latter study, however, reported a "clear and unambiguous sexual dimorphism
within the major phases of the EOD itself" (p. 400), while Lucker and Kramer (1981) did not find any EOD‑related
sex difference in this species. No studies have yet investigated the
possibility of hormonal control of the EOD in this species.
The EOD
of weakly discharging electric fish proves to be an excellent behavior to study
behavior‑endocrine interactions and behavioral physiology. EODs provide a
clear example of behavioral variability as a function of species, sex and of
behavioral plasticity in response to changing environmental conditions. Many of
the studies on these animals have been performed without adequate scientific
designs or were confounded by captivity or other factors which resulted in
disparate findings. But the plasticity of the EOD in response to a multitude of
variables has led to the development of a viable model for the study of the
effects of captivity on reproductive and behavioral physiology in vertebrates.
344 Sex, hormones, environment and the
captivity model
References
D.H.
Taylor and S.I. Guttman), Plenum Press,
Assem, H. and Hanke,
W. (1981) Cortisol and osmotic adjustment of the euryhaline
teleost, Sarotherodon mossambicus. Gen. Comp. Endocrinol.
43, 370‑80.
Bass,
A.H. (1986c) Species differences in electric organs of mormyrids: substrates
for species‑typical electric organ discharge waveforms. J. Comp.
Neurol. 244, 313‑30.
Bass,
A.H. and Hopkins, C.D. (1983) Hormonal control of sexual differentiation:
changes in electric organ discharge waveform. Science 220, 971‑4.
Bass,
A.H. and Hopkins, C.D. (1984) Shifts in frequency tuning of electroreceptors
in androgen ‑treated mormyrid fish. J.
Comp. Physiol. 15 5A, 713‑24.
Bass,
A.H. and Hopkins, C.D. (1985) Hormonal control of sex differences in the
electric organ discharge (EOD) of mormyrid fishes. J. Comp. Physiol. 156A, 587604.
Bass,
A.H. and Volman, S.F. (1987) From behavior to membranes: testosterone-induced
changes in action potential duration in electric organs. Proc. Natn. Acad. Sci. USA 84, 92958.
Bass,
A.H., Segil, N. and Kelley, D.B. (1984) A steroid sensitive electromotor
pathway in mormyrid fish: electric organ morphology, androgen receptor
biochemistry and steroid autoradiography.
Soc. Neurosci. Abstr. 10, 927.
Bass,
A.H., Denizot, J.‑P. and Marchaterre, M.A. (1986a) Ultrastructural
features and hormone‑dependent sex differences of mormyrid electric
organs. J. comp. Neurol. 254, 511‑28.
Bass,
A.H., Segil, N. and Kelley, D.B. (1986b) Androgen binding in the brain and
electric organ of a mormyrid fish. J.
Comp. Physiol. 159A, 535‑44.
Bennett,
M.V.L. (1971a) Electric organs, in Fish
Physiology, Vol. V (eds W.S. Hoar and D.J. Randall), Academic Press,
Bennett,
M.V.L. (1971b) Electroreception, in Fish
Physiology, Vol. V (eds W.S. Hoar and D.J. Randall), Academic Press,
Bratton, B.C. and Kramer, B. (1988)
Intraspccific variability of the pulse‑type discharges of the African
electric fishes, Pollimyrus isidori and Petrocephalus bovei (Mormyridae, Teleostei), and their dependence
on water conductivity. Exp. Biol. 47, 227‑38.
Crawford,
J.D. (1992) Individual and sex specificity in the electric organ discharges of
breeding mormyrid fish (Pollimyrus
isidori). 1. Exp. Biol. 164, 79‑102.
Elton.
R.H. (1979) Baboon behaviour under crowded conditions, in Captivity and Behaviour (eds J. Erwin, T.L. Maple and G. Mitchell),
Van
References
345
Erwin,
J. and Deni, R. (1979) Strangers in a strange land: abnormal behaviours or
abnormal environments? In Captivity and
Behaviors (eds. J. Erwin, T.I. Maple and G. Mitchell). Van
Ferrari,
M.B. and Zakon, H.H. (1993) Conductances contributing
to the action potential of Sternopygus electrocytes. J. Comp. Physiol. 173A,
28 1‑92.
Freedman,
E.G., Olyarchuk, J., Marchaterre, M.A. and Bass,
A.11. (1989) A temporal analysis of
testosterone‑induced changes in electric organs and electric organ discharges
of mormyrid fishes. J. Ncurobiol. 20, 619‑34.
Hagedorn,
M. and Carr, C. (1985) Single electrocytcs produce a sexually dimorphic signal
in South American electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae).
J. Comp. Physiol. 156A, 511‑23.
Hagedorn,
M. and Heiligenberg, W. (1985) Court and spark: electric signals in the
courtship and mating of gymnotoid fish. Anim. Behav. 33, 254‑65.
Hannes, R.P. and Franck, 1). (1983) Diurnal
variation of blood androgen and corticoid levels in male swordtails Xiphophorus helleri. Zool.
Jb. Physiol. 87,
337‑41.
Harding,
C.F. (1986) The role of androgen metabolism in the activation of male behavior,
in Reproduction: A Behavioral and
Neuroendocrine Perspective. Ann. N. Y. Acad. Sci., Vol. 474 (eds B.R. Komisaruk, H.F. Siegel., M.‑F. Cheng and H.H. Feder),
Hopkins,
C.D. (1981a) On the diversity of electric signals in a community of mormyrid
electric fish in
Ilan.
Z. and Yaron. Z. (1983) Interference of o,p'DDD with interrenal function and cortisol metabolism in Sarotherodon aureus (Steindachner). J.
Fish Biol. 22, 657‑69.
Keppel,
G. (1982) Design and Analysis. A
Researcher's Handbook. 2nd edn, Prentice Hall,
Englewood‑Cliffs, NJ. (see pp. 15 5‑6).
Kirschbaum,
F. (1983) Myogenic electric organ precedes the neurogcnic organ in Apteronotid
fish. Naturwissenschaften 70,
205‑7.
346 Sex,
hormones, environment and the captivity model
Kramer,
B. (1976a) The attack frequency of Gnathonemus
petersii towards electrically silent (denervated) and intact conspecifics,
and towards another mormyrid (Brienomyrus
Kramer,
B. (1976b) Electric signalling during aggressive behavior in Mormyrus rume (Mormyridae, Teleostci). Naturwissenschaften 63, 48‑9.
Kramer,
B. and Otto, B. (1988) Female discharges are more electrifying: spontaneous
preference in the electric fish, Eigenmannia
(Gymnotiformes, Teleostei). Behav. Ecol.
Sociobiol. 23, 55‑‑60.
Kramer,
B. and Wcstby, G.W.M. (1985) No sex difference in the wavcform of the pulse
type electric fish, Gnathonemus petersii (Mormyridae).
Experientia 41,
153031.
Landsman,
R.E. (1990) Seasonal, hormonally~controlled,
phase-specific sex differences in electric organ discharges of the weakly
electric fish, Gnathonemus petersii (Morrnyridae),
and the effects of captivity on these discharges (see also Psychol. Diss. Abstr., 1990, 1135
B), Phl) thesis, The City University of New York, New
York, 272 pp.
Landsman,
R.E. (1991) Captivity affects behavioral physiology: plasticity in signalling
sexual identity. Experientia 47,
11‑8.
Landsman,
R.E. ( 1993a) The effects of captivity on the electric organ discharge and
plasma hormone levels in Gnathonemus
petersii (Mormyriformes). J.
Comp. Physiol. 172A, 619‑‑31.
Landsman.
R.E,. ( 1993b) Sex differences in external morphology and electric organ
discharges in imported Gnathonemus
petersii (Mormyriformes). Anim. Behav. 46, 417-29.
Landsman,
R.E. and Moller, P (1988) Testosterone changes the electric organ discharge and
external morphology of the mormyrid fish, Gnathonemus
petersii (Mormvriformes).
Experientia 44,
900‑‑3.
Landsman,
R.E. and Moller, P. (1991) Laboratory captivity reverses sex differences in
electric organ discharge in mormyrid fish, in Proc. Fourth 1nt. Symp. Reprod. Physiol. Fish 1991 (eds A.P. Scott, J.P. Sumpter,
D.E. Kime and M.S. Rolfe),
Landsman.
R.E. and Moller, P. (1993) Captivity and signal plasticity in mormyrid fish
communication, in Contributions of' Electrosensory Systerns to
Neurobiology and Neurocthology. Proceedings of a
Conference in Honor of the Scientific Career of Thomas Szabo
(eds C.C. Bell, C.D. Hopkins and K. Grant), J.
Comp. Physiol. 173A, 17 2 ‑3.
Landsman,
R.E.. ]ou. S.H. and Moller, P. (1987) Stress obscures
signalling of sexual identity in Gnathonemus
petersii (Mormyriformes), in Proc.
Third Int. Symp. Reprod. Physiol. Fish 1987 (eds D.R. Idler. L.W. Crim and
J.M. Walsh),
Landsman,
R.E., Harding. C,F.. Moller, P. and Thomas, P. (1990) The effects of androgens
and estrogens on the external morphology and electric organ discharge waveform
of Gnathonemus petersii (Mormyridae, Teleostei). Horm. Behav. 24, 5 32‑‑ 5 3.
Mazeaud, M.M. and Mazeaud,
F. (1981) Adrenergic responses to stress in fish, in Stress and Fish (ed. A. D.
Pickering), Academic Press, New York, pp. 49‑75.
References
347
Mazeaud, M.M., Mazeaud,
F. and Donaldson, E.M. (1977) Primary and secondary effects of stress in fish:
some new data with a general review. Trans.
Am. Fish. Soc. 3, 201‑12.
Meyer,
J.H. (1983) Steroid influences upon the discharge frequencies of a weakly
electric fish. J. Comp. Physiol. 153A, 29‑37.
Meyer,
J.H. (1984) Steroid influences upon discharge frequencies of' intact and
isolated pacemakers of weakly electric fish. J. Comp. Physiol. 154A, 659‑ 68.
Meyer,
J.H., Zakon, H.H. and Heiligenberg. W. (1984) Steroid influences upon the electrosensory system of weakly electric fish: direct
effects upon discharge frequencies with indirect effects upon electroreceptor
tuning. J. Comp. Physiol. 154A, 625‑‑31.
Meyer,
J.H., Leong, M. and Keller, CIL (1987) Hormone‑induced
and ontogenetic changes in electric organ discharge and electroreceptor tuning
in the weakly electric fish Apteronotus.
1. Comp. Physiol. 160A, 383‑‑94.
Miller,
Lj and ‑Moore, F.L. (1983) Intracranial
administration of corticolropin‑like peptides
increases incidence of amphibian reproductive behaviour. Peptides 4, 729‑311.
Mills,
A. and Zakon, H.H. (1987) Coordination of' EOD frequencv
arid pulse duration in weakly electric wave fish: the influence of androgens. J. Comp. Physiol. 161 A, 41 '‑30.
Mills,
A., Zakon, H.H., Marchatcrre, M.A. and Bass, A.H.
(1992) Electric organ morphology of Sternopygus
macrurus, a wave‑type weakly electric fish with a sexually dimorphic
EOD. J. Neurobiol.
23. 920‑11
Moller,
P. (1980) Electroperception. Oceanus 23, 44‑54.
Moore,
F.L. and Deviche, P. (1988) Neuroendocrine processing
of environmental information in amphibians, in Processing of Environmental
Information in Vertebrates (ed. M.H. Statson),
Springer, New York, pp. 20-45.
Moore,
M.C. and Marler,
Noakes, D.L.G. and Leatherland,
I.F. (1977) Social dominance and interrenal cell
activity in rainbow trout Salmo gairdneri. Env.. Biol. Fishes 2,
131‑6.
348 Sex,
hormones, environment and the captivity model
Peters,
G., Delvanthal, H. and Klinger, M. (1980)
Physiological and morphological effects of social stress in the cc],
Rivier, C., Rivier, 1. and Vale, W. (1986) Stress‑induced inhibition of reproductive
functions: role of endogenous corticotropin‑releasing
factor. Science 231, 607‑9.
Safford,
S.E. and Thomas, P. (1987) Effects of capture and handling on circulating
levels of gonadal steroids and cortisol in the spotted trout, in Reproductive Physiology of Fish (eds D.R. Edler
and J.M. Walsh), University of Newfoundland, St. Johns, pp. 312.
Strange,
R.I., Schreck, C.B. and Golden, J.T. (1977) Corticoid
stress responses to handling and temperature in salmonida.
Trans. Arn.
Fish. Soc. 106, 213‑8.
Truscott,
B., Walsh, J.M.,
Westby,
G.W.M. and Kirschbaum, F. (1977) Emergence and development of the electric
organ discharge in the mormyrid fish, Pollimyrus
isidori. 1. The larval discharge. J.
Comp. Physiol. 122A, 251‑71.
Westby,
G.W.M. and Kirschbaum, F. (1981) Sex differences in the electric organ
discharge of Eigenmannia virescens
and the effect of gonadal maturation, in Advances
in Physiological Sciences. Vol. 31: Sensory Physiology of Aquatic Lower
Vertebrates (eds T. Szabo and G. Cz6h), Pergamon Press, Akademiai Kiado.
Westby,
G.W.M. and Kirschbaum, F. (1982) Sex differences in the waveform of the pulse‑type
electric fish. Pollimyrus isidori (Mormyridac). J. Comp.
Physiol. 145A, 399‑403.
Wingfield, J.C. (1988) Changes in reproductive
function of free‑living birds in direct response to environmental
perturbations, in Processing of E'nvironmental Inj6rination in Vertebrates (ed. M.H.
Stetson), Springer,
Zakon,
H.H. (1986) The electroreceptivc periphery, in Electroreception (eds T.H. Bullock and
W. Heiligenberg), Wiley,
Zakon,
H.H. (1993) Weakly electric fish as model systems for studying long‑term
steroid action on neural circuits. Brain
Behav. Evol. 42,
242‑51.
Zakon,
H.H., Mills, A.C. and Ferrari, M.B. (1991a) Androgen‑dependent modulation
of the electroscnsory and electromotor systems of a
weakly electric fish. Sem. Neurosci. 3, 449‑57.
Zakon,
H.H., Thomas, P. and Yan, H.Y. (199 lb) Electric
organ discharge frequency and plasma sex steroid levels during gonadal
recrudescence in a natural population of Sternopygus
macrurus. J. Comp. Physiol. 169A, 49 3 ‑9.
Zoeller, R.T. and